

First-in-disease therapies for patients with rare genetic skin diseases Q1 2025 Financial Results & Corporate Update May 15, 2025

Forward Looking Statements This presentation contains forward-looking statements of Palvella Therapeutics, Inc. (the Company”) within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements that are not historical facts, and in some cases, can be identified by terms such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained in this presentation include, but are not limited to, statements regarding the Company’s future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters the Company’s current and prospective product candidates, the Company's planned research and development activities, the Company's planned clinical trials, including timing of receipt of data from the same, the planned regulatory framework for the Company's product candidates, the strength of the Company's intellectual property portfolio, and projections of the Company’s future financial results and other metrics. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon current estimates and assumptions of the Company and its management and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: competition, the ability of the company to grow and manage growth, maintain relationships with suppliers and retain its management and key employees; the success, cost and timing of the Company’s product development activities, studies and clinical trials; changes in applicable laws or regulations; the possibility that the Company may be adversely affected by other economic, business or competitive factors; the Company’s estimates of expenses and profitability; the evolution of the markets in which the Company competes; the ability of the Company to implement its strategic initiatives and continue to innovate its existing products; and the ability of the Company to defend its intellectual property. Nothing in this Presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. The Company undertakes no duty to update these forward-looking statements. Industry and Market Data The Company may from time to time provide estimates, projections and other information concerning its industry, the general business environment, and the markets for certain conditions, including estimates regarding the potential size of those markets and the estimated incidence and prevalence of certain medical conditions. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties, and actual events, circumstances or numbers, including actual disease prevalence rates and market size, may differ materially from the information reflected in this presentation. Unless otherwise expressly stated, we obtained this industry, business information, market data, prevalence information and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data, and similar sources, in some cases applying our own assumptions and analysis that may, in the future, prove not to have been accurate. Trademarks This Presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the TM, SM © or ® symbols, but the Company will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade names and copyrights.

Photo credit: Nasdaq, Inc./ Vanja Savic.
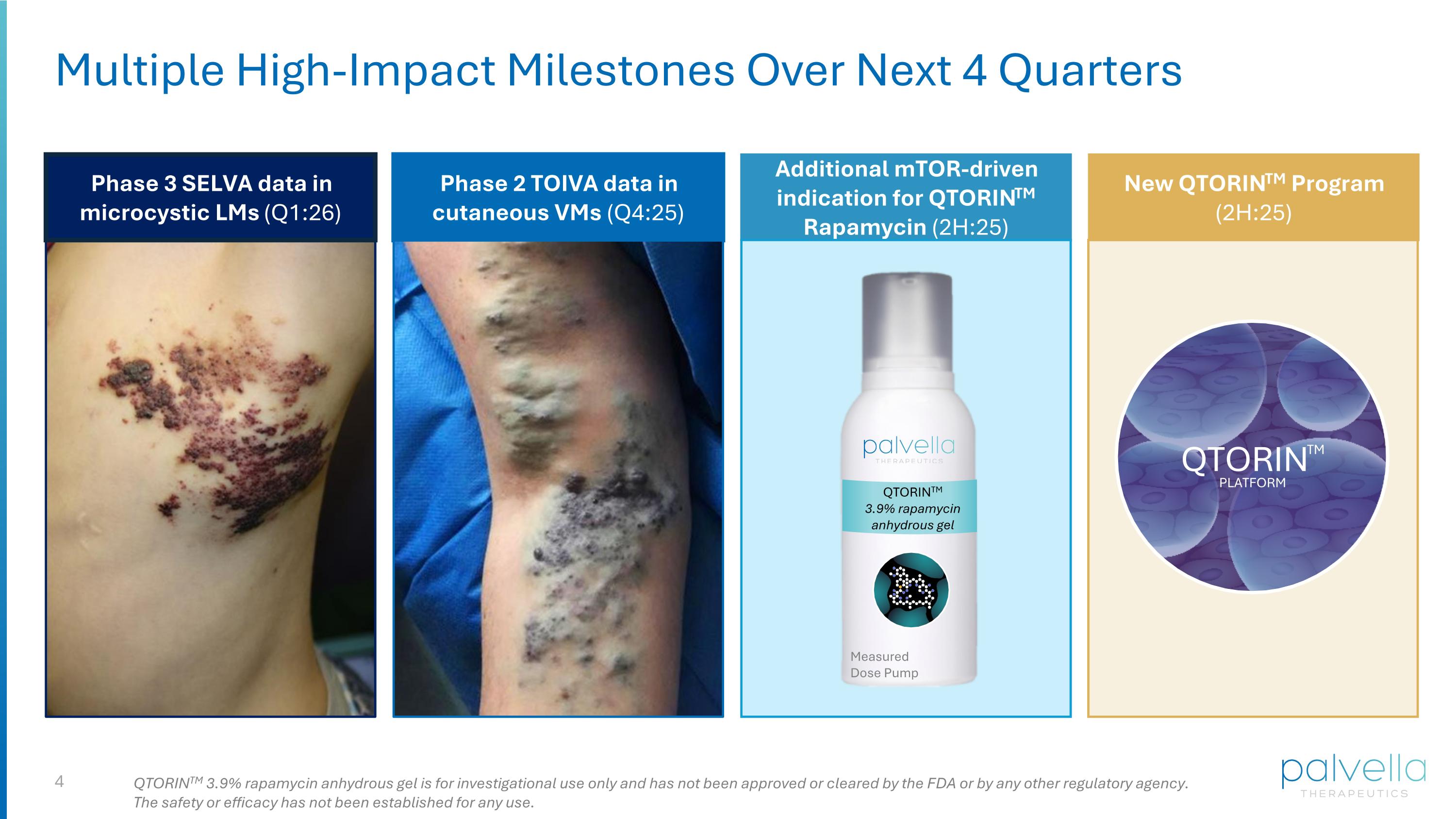
Multiple High-Impact Milestones Over Next 4 Quarters Phase 3 SELVA data in microcystic LMs (Q1:26) Phase 2 TOIVA data in cutaneous VMs (Q4:25) Additional mTOR-driven indication for QTORINTM Rapamycin (2H:25) New QTORINTM Program (2H:25) QTORINTM QTORINTM PLATFORM QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use. QTORINTM 3.9% rapamycin anhydrous gel Measured Dose Pump
Continued Strong Momentum at Palvella QTORINTM rapamycin for microcystic LMs: Phase 3 SELVA trial Exceeded enrollment target of 40 patients; enrollment expected to close in June 2025 Top-line readout anticipated Q1 2026 Phase 2 TOIVA study on track in cutaneous VMs 6 sites open and enrolling Top-line readout anticipated Q4 2025 Insights from SID Meeting and ISSVA Conference: support significant unmet need and attractive commercial opportunity in microcystic LMs Fortifying leadership team in anticipation of potential U.S. commercialization Hired Jason Burdette as SVP, CMC & Technical Operations (Jan 2025) Chief Commercial Officer recruitment ongoing; planned hire in 2H 2025 Strengthening patent position: 5th U.S. patent issuance with claims into 2038 Patents augmented by trade secrets and anticipated seven-year orphan exclusivity LMs: lymphatic malformations. VMs: venous malformations. SID: Society of Investigative Dermatology. ISSVA: International Society for the Study of Vascular Anomalies.

QTORINTM 3.9% RAPAMYCIN Microcystic Lymphatic Malformations FOR
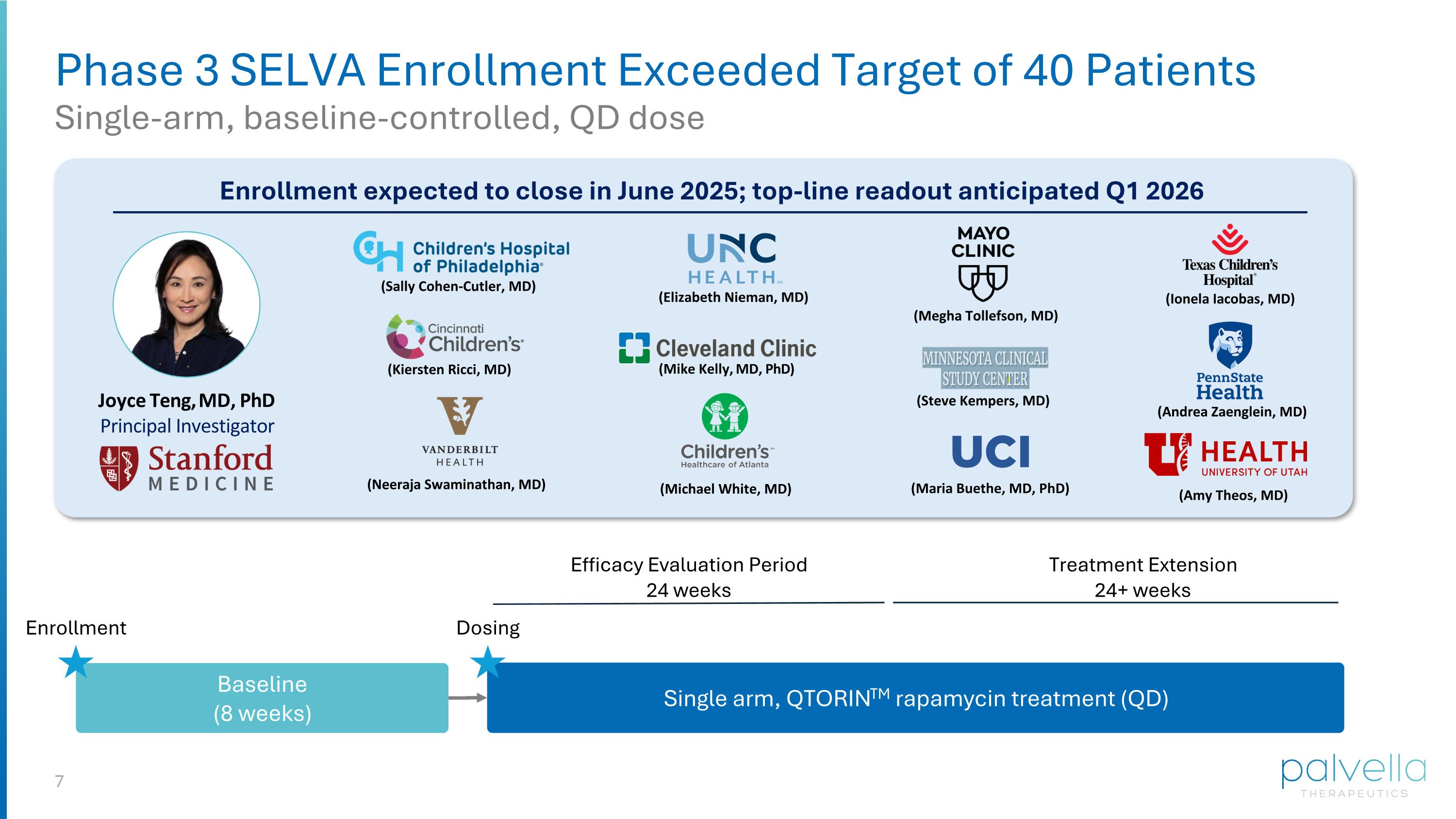
Phase 3 SELVA Enrollment Exceeded Target of 40 Patients Single-arm, baseline-controlled, QD dose (Mike Kelly, MD, PhD) (Kiersten Ricci, MD) (Megha Tollefson, MD) (Sally Cohen-Cutler, MD) (Elizabeth Nieman, MD) (Ionela Iacobas, MD) (Andrea Zaenglein, MD) (Steve Kempers, MD) Enrollment expected to close in June 2025; top-line readout anticipated Q1 2026 Joyce Teng, MD, PhD Principal Investigator (Neeraja Swaminathan, MD) (Michael White, MD) (Amy Theos, MD) (Maria Buethe, MD, PhD) Single arm, QTORINTM rapamycin treatment (QD) Treatment Extension 24+ weeks Efficacy Evaluation Period 24 weeks Baseline (8 weeks) Enrollment Dosing
QTORINTM Rapamycin for Treatment of mLMs: Regulatory Overview Consistent and productive engagement with FDA on development program FDA Overview: Center: Center for Drug Evaluation Research (CDER) Division: Dermatology and Dentistry Division Leadership: Dr. Jill Lindstrom remains in Director role NDA Review and Signature: Due to planned 505(b)(2) pathway, division leadership is responsible for NDA decision Palvella is anticipating expedited pathway to submission given Breakthrough, Fast Track and Orphan Drug Designations and 505(b)(2) pathway QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use. Out of 51 grant applications received by the FDA Orphan Products Grants Program in fiscal year 2024, Palvella’s clinical trial was one of seven new clinical trials and only Phase 3 program that was awarded a grant (up to $2.6 million) Other FDA dynamics for pipeline programs New potential accelerated pathway for rare and ultra rare disease drugs based on a “plausible mechanism” announced by Commissioner Makary
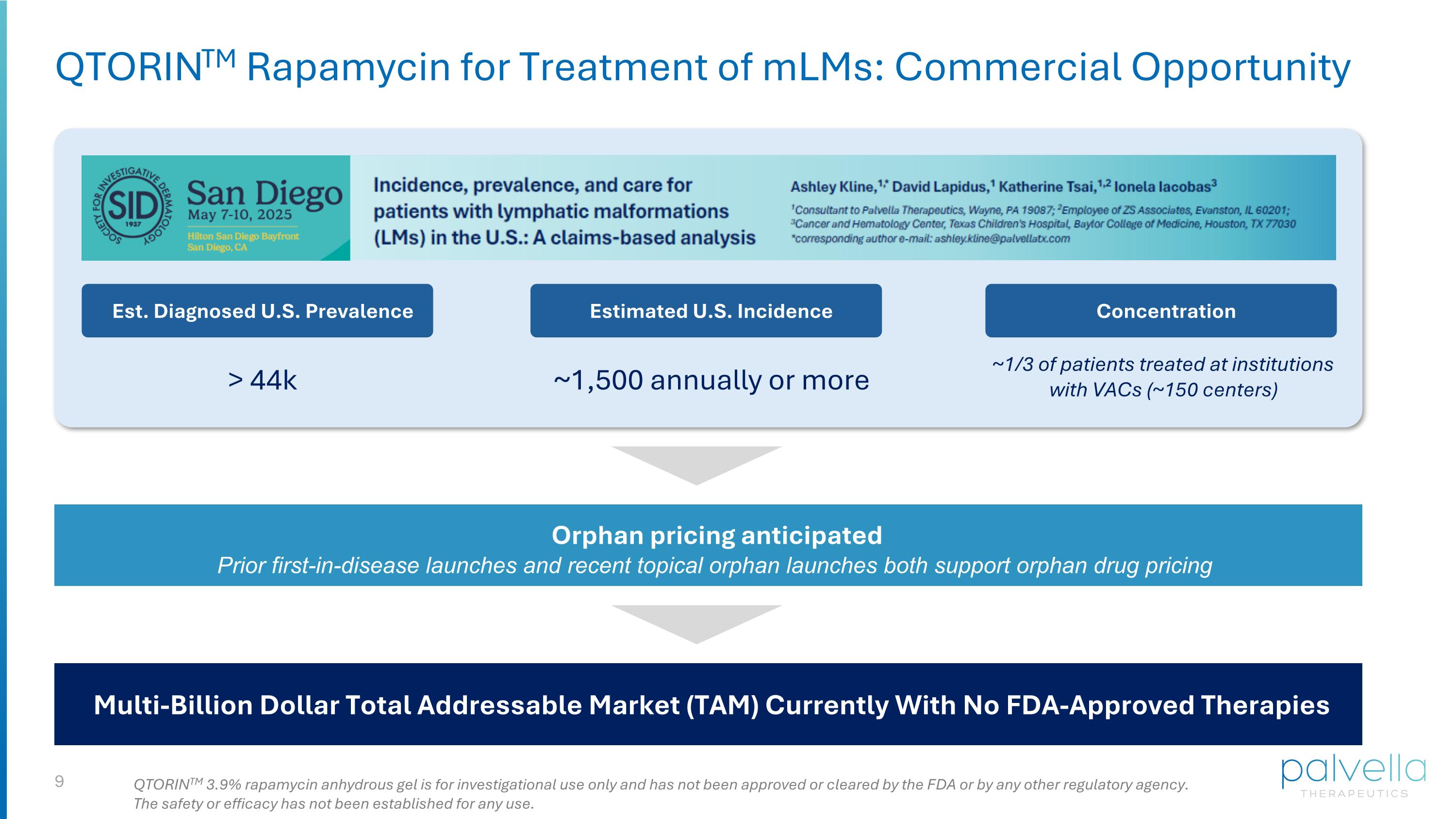
QTORINTM Rapamycin for Treatment of mLMs: Commercial Opportunity Est. Diagnosed U.S. Prevalence QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use. Estimated U.S. Incidence Concentration Multi-Billion Dollar Total Addressable Market (TAM) Currently With No FDA-Approved Therapies Orphan pricing anticipated Prior first-in-disease launches and recent topical orphan launches both support orphan drug pricing ~1/3 of patients treated at institutions with VACs (~150 centers) ~1,500 annually or more > 44k

ISSVA Conference 2025: April 23rd-25th in Paris, France Treatment paradigm rapidly evolving to targeted pharmacotherapy approaches, replacing surgery and sclerotherapy Off-label systemic agents, incl. oral PI3K inhibitors, introduce unacceptable side effects (e.g., growth retardation) for diseases that locally present on the skin – significant unmet needs exist for targeted, topical therapies Strong KOL support for QTORINTM Rapamycin and other potential QTORINTM programs Identification of additional high unmet need clinical indications that could be future disease targets for Palvella

QTORINTM 3.9% RAPAMYCIN Cutaneous Venous Malformations FOR
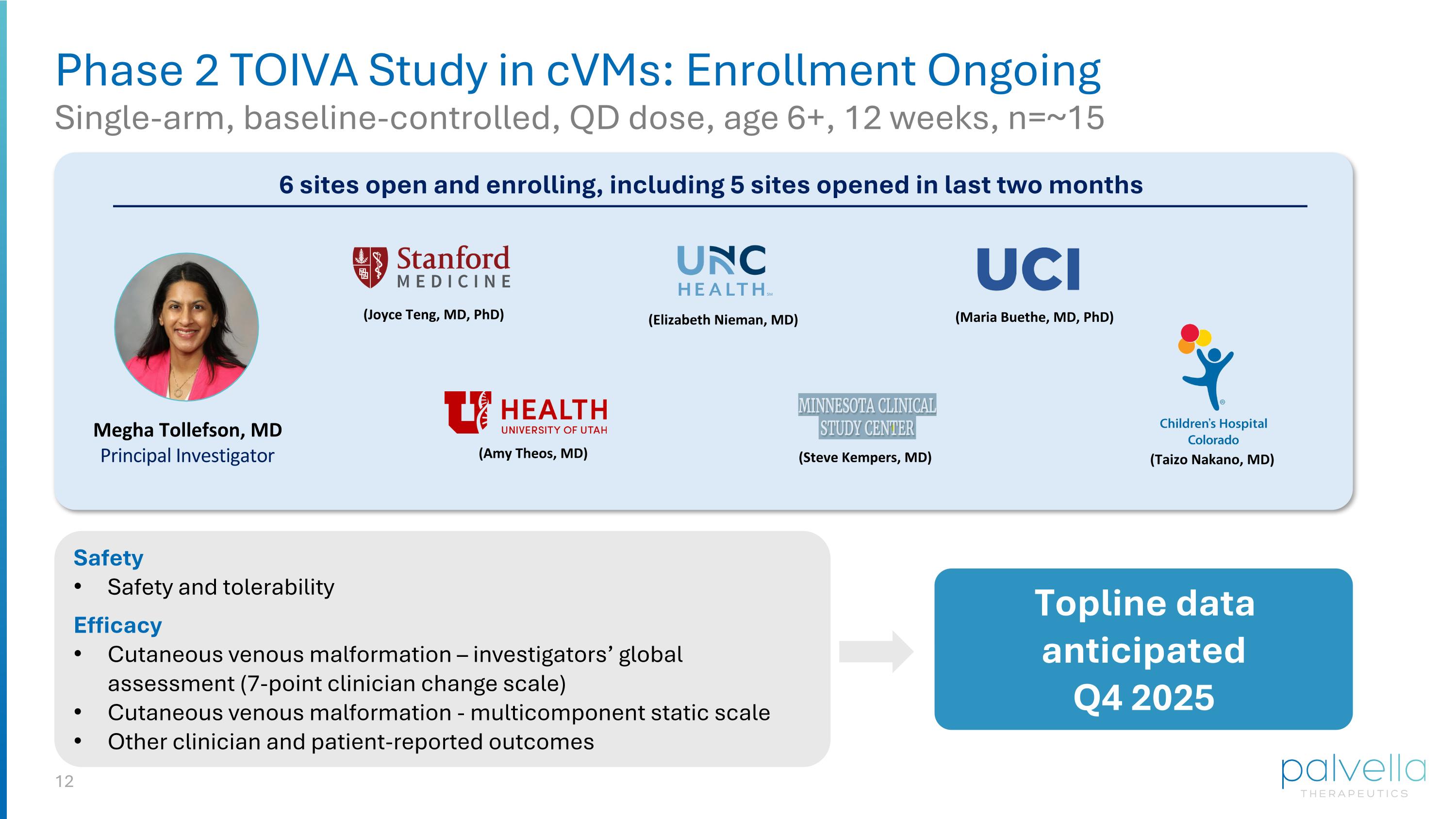
Phase 2 TOIVA Study in cVMs: Enrollment Ongoing Single-arm, baseline-controlled, QD dose, age 6+, 12 weeks, n=~15 (Elizabeth Nieman, MD) Safety Safety and tolerability Efficacy Cutaneous venous malformation – investigators’ global assessment (7-point clinician change scale) Cutaneous venous malformation - multicomponent static scale Other clinician and patient-reported outcomes Topline data anticipated Q4 2025 Megha Tollefson, MD Principal Investigator 6 sites open and enrolling, including 5 sites opened in last two months (Steve Kempers, MD) (Joyce Teng, MD, PhD) (Amy Theos, MD) (Maria Buethe, MD, PhD) (Taizo Nakano, MD)
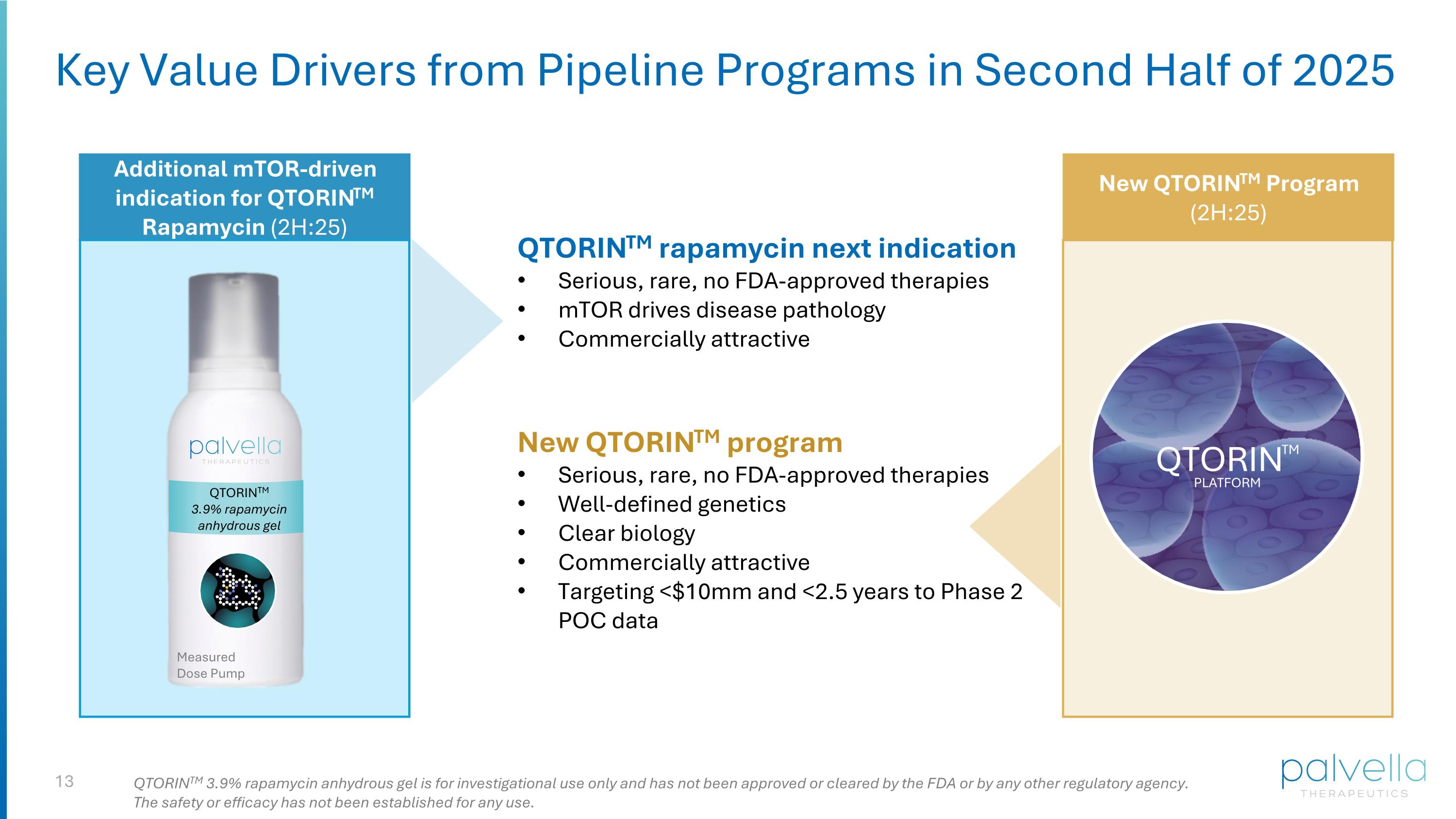
Key Value Drivers from Pipeline Programs in Second Half of 2025 Additional mTOR-driven indication for QTORINTM Rapamycin (2H:25) New QTORINTM Program (2H:25) QTORINTM QTORINTM PLATFORM QTORINTM 3.9% rapamycin anhydrous gel QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use. QTORINTM rapamycin next indication Serious, rare, no FDA-approved therapies mTOR drives disease pathology Commercially attractive New QTORINTM program Serious, rare, no FDA-approved therapies Well-defined genetics Clear biology Commercially attractive Targeting <$10mm and <2.5 years to Phase 2 POC data Measured Dose Pump

Financial Update
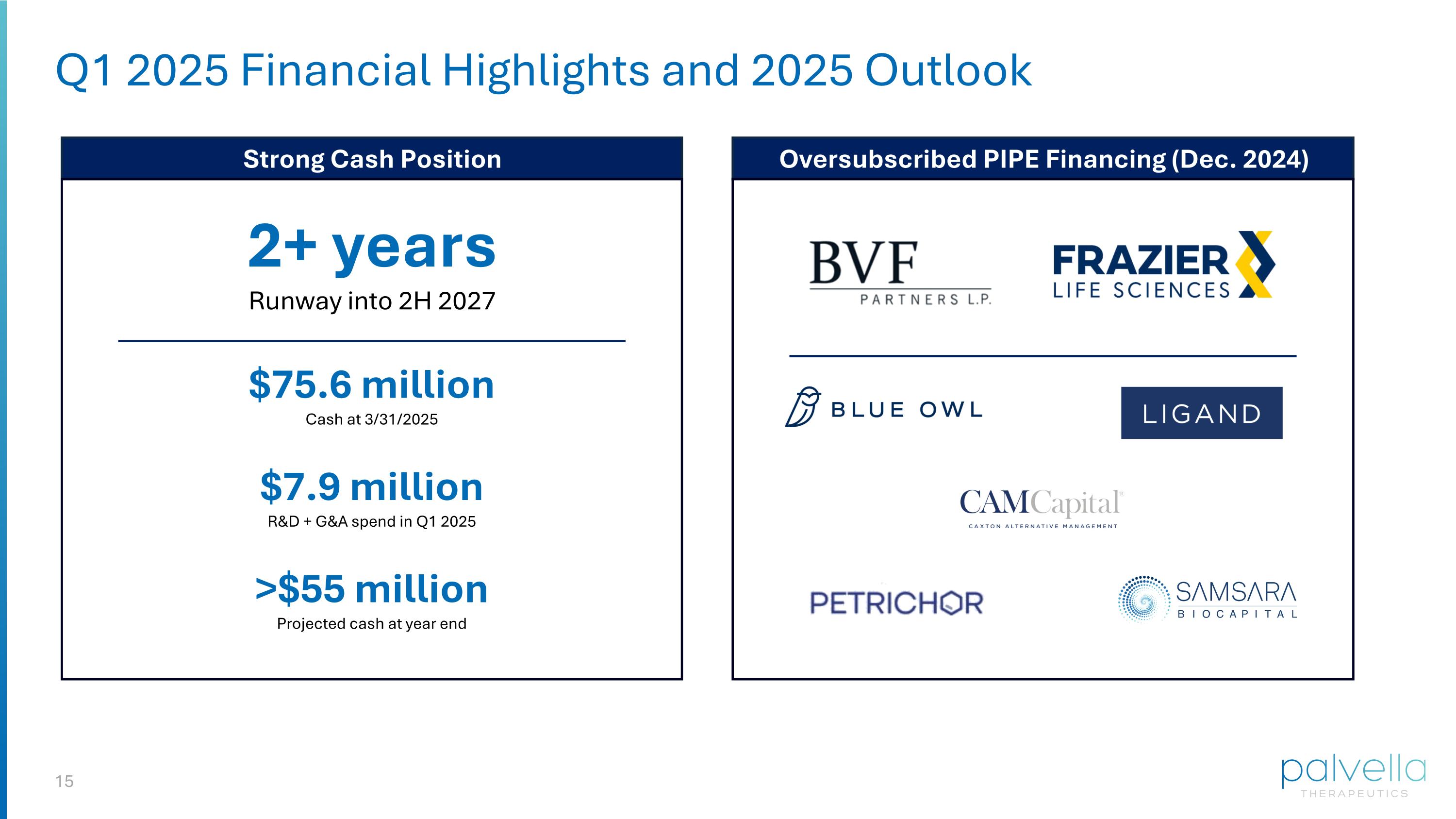
Q1 2025 Financial Highlights and 2025 Outlook 2+ years Runway into 2H 2027 $75.6 million Cash at 3/31/2025 $7.9 million R&D + G&A spend in Q1 2025 >$55 million Projected cash at year end Oversubscribed PIPE Financing (Dec. 2024) Strong Cash Position

Q&A Striving to be first for rare disease patients