UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
| |
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2018
OR
|
| |
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-37471
PIERIS PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
| | |
Nevada | | EIN 30-0784346 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
255 State Street, 9th Floor Boston, MA United States | | 02109 |
(Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code
857-246-8998
Securities registered pursuant to Section 12(b) of the Exchange Act:
|
| | |
Title of each class | | Name of each exchange on which registered |
Common Stock, par value $0.001 per share | | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act:
None
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ý No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
| | | | | | |
Large accelerated filer | | ☐ | | Accelerated filer | | ý
|
| | | |
Non-accelerated filer | | ☐ | | Smaller reporting company | | ý
|
| | | | | | |
Emerging growth company ý | | | | |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ý
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant on June 29, 2018, the last business day of the registrant’s most recently completed second fiscal quarter, based on the closing price on that date of $5.07, was $273,283,424.
As of March 11, 2019, the registrant had 49,151,219 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
TABLE OF CONTENTS
Forward Looking Statements
This annual report on Form 10-K for the year ended December 31, 2018, or this Annual Report on Form 10-K, contains forward looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that involve risks and uncertainties, principally in the sections entitled “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” All statements other than statements of historical fact contained in this Annual Report on Form 10-K, including statements regarding future events, our future financial performance, business strategy and plans and objectives of management for future operations, are forward-looking statements. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “ongoing,” “could,” “estimates,” “expects,” “intends,” “may,” “appears,” “suggests,” “future,” “likely,” “goal,” “plans,” “potential,” “projects,” “predicts,” “should,” “would” or “will” or the negative of these terms or other comparable terminology. Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we caution you that these statements are based on our projections of the future that are subject to known and unknown risks and uncertainties and other factors that may cause our actual results, level of activity, performance or achievements expressed or implied by these forward-looking statements, to differ. The description of our Business set forth in Item 1, the Risk Factors set forth in Item 1A and our Management’s Discussion and Analysis of Financial Condition and Results of Operations set forth in Item 7 as well as other sections in this report, discuss some of the factors that could contribute to these differences. These forward-looking statements include, among other things, statements about:
| |
• | the accuracy of our estimates regarding expenses, future revenues, uses of cash, capital requirements and the need for additional financing; |
| |
• | the initiation, cost, timing, progress and results of our development activities, preclinical studies and clinical trials; |
| |
• | the timing of and our ability to obtain and maintain regulatory approval of our existing product candidates, any product candidates that we may develop, and any related restrictions, limitations and/or warnings in the label of any approved product candidates; |
| |
• | our plans to research, develop and commercialize our current and future product candidates; |
| |
• | our collaborators’ election to pursue research, development and commercialization activities; |
| |
• | our ability to obtain future reimbursement and/or milestone payments from our collaborators; |
| |
• | our ability to attract collaborators with development, regulatory and commercialization expertise; |
| |
• | our ability to obtain and maintain intellectual property protection for our product candidates; |
| |
• | our ability to successfully commercialize our product candidates; |
| |
• | the size and growth of the markets for our product candidates and our ability to serve those markets; |
| |
• | the rate and degree of market acceptance of any future products; |
| |
• | the success of competing drugs that are or become available; |
| |
• | regulatory developments in the United States and other countries; |
| |
• | the performance of our third-party suppliers and manufacturers and our ability to obtain alternative sources of raw materials; |
| |
• | our ability to obtain additional financing; |
| |
• | our use of the proceeds from our securities offerings; |
| |
• | any restrictions on our ability to use our net operating loss carryforwards; and |
| |
• | our ability to attract and retain key personnel. |
Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time and it is not possible for us to predict all risk factors, nor can we address the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause our actual results to differ materially from those contained in any forward-looking statements.
You should not place undue reliance on any forward-looking statement, each of which applies only as of the date of this Annual Report on Form 10-K. Before you invest in our securities, you should be aware that the occurrence of the events described in the section entitled “Risk Factors” and elsewhere in this Annual Report on Form10-K could negatively affect our business, operating results, financial condition and stock price. All forward-looking statements included in this document are based on information available to us on the date hereof, and except as required by law, we undertake no obligation to update or revise publicly any of the forward-looking statements after the date of this Annual Report on Form 10-K to conform our statements to actual results or changed expectations.
We have registered trademarks for Pieris® and Anticalin®. All other trademarks, trade names and service marks included in this Annual Report on Form10-K are the property of their respective owners. Use or display by us of other parties’ trademarks, trade dress or products is not intended to and does not imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owner.
As used in this Annual Report on Form10-K, unless the context indicates or otherwise requires, “our Company”, “the Company”, “Pieris”, “we”, “us” and “our” refer to Pieris Pharmaceuticals, Inc., a Nevada corporation, and its consolidated subsidiary, Pieris Pharmaceuticals GmbH (formerly known as Pieris AG), a company organized under the laws of Germany, Pieris Australia Pty Ltd., a company organized under the laws of Australia that is a consolidated subsidiary of Pieris Pharmaceuticals GmbH, and Pieris Pharmaceuticals Securities Corporation, a Massachusetts securities corporation, a consolidated subsidiary of Pieris Pharmaceuticals, Inc. Effective as of August 26, 2015 and with notification from the Amtsgericht München as of September 29, 2015, Pieris AG was transformed to Pieris Pharmaceuticals GmbH as a result of a change in the legal entity.
Currency Presentation and Currency Translation
Unless otherwise indicated, all references to “dollars,” “$,” “US $” or “US dollars” are to the lawful currency of the United States. All references in this Report to “euro” or “€” are to the currency introduced at the start of the third stage of the European Economic and Monetary Union pursuant to the Treaty establishing the European Community, as amended. We prepare our financial statements in US dollars.
The functional currency for our operations is primarily the euro. With respect to our financial statements, the translation from the euro to US dollars is performed for balance sheet accounts using exchange rates in effect at the balance sheet date and for revenue and expense accounts using a weighted average exchange rate during the period. The resulting translation adjustments are recorded as a component of accumulated other comprehensive loss.
Where in this Report we refer to amounts in euros, we have for your convenience also, in certain cases, provided a conversion of those amounts to US dollars in parentheses. Where the numbers refer to a specific balance sheet account date or financial statement account period, we have used the exchange rate that was used to perform the conversions in connection with the applicable financial statement. In all other instances, unless otherwise indicated, the conversions have been made using the noon buying rate of €1.00 to US $1.1450 based on Thomson Reuters as of December 31, 2018.
PART I
Corporate History
General
Pieris Pharmaceuticals, Inc. was incorporated in the State of Nevada in May 2013 under the name “Marika Inc.” Pieris Pharmaceuticals, Inc. began operating the business of Pieris Pharmaceuticals GmbH, or Pieris GmbH, through a reverse acquisition on December 17, 2014. Pieris GmbH (formerly Pieris AG, a German company which was founded in 2001) continues as an operating subsidiary of Pieris Pharmaceuticals, Inc.; Pieris Pharmaceuticals, Inc. is the sole stockholder of Pieris GmbH.
Pieris Pharmaceuticals, Inc.'s corporate headquarters are located at 255 State Street, 9th Floor, Boston, Massachusetts 02109. The research facilities of Pieris GmbH are located in Freising, Germany. Beginning in late 2019 we anticipate that the research facilities of Pieris GmbH will be relocated to Hallbergmoos, Germany. Pieris Australia Pty Ltd., a wholly-owned subsidiary of Pieris GmbH, was formed on February 14, 2014 to conduct research and development activities in Australia. Pieris Pharmaceuticals Securities Corporation, a wholly-owned subsidiary of Pieris Pharmaceuticals, Inc. was formed on December 14, 2016 to buy, sell, deal in, or hold securities on its own behalf and not as a broker, and will engage in its activities exclusively for investment purposes.
Business Overview
We are a clinical-stage biotechnology company that discovers and develops Anticalin-based drugs to target validated disease pathways in unique and transformative ways. Our clinical pipeline includes an inhaled IL-4 receptor alpha, or IL-4Rα, targeting Anticalin protein to treat asthma and an immuno-oncology, or IO, bispecific protein targeting HER2 and 4-1BB. Proprietary to us, Anticalin proteins are a novel class of clinically-tested therapeutics validated by partnerships with leading pharmaceutical companies.
Anticalin proteins are a class of low molecular-weight therapeutic proteins derived from lipocalins, which are naturally occurring proteins typically found in human blood plasma and other bodily fluids. Anticalin proteins function similarly to monoclonal antibodies by binding tightly and specifically to a diverse range of targets. An antibody is a large protein used by the immune system that recognizes a unique part of a target molecule, called an antigen. We believe Anticalin proteins possess numerous advantages over antibodies in certain applications. For example, Anticalin proteins are relatively small in size and monomeric, meaning they are comprised of a single polypeptide rather than a multi-polypeptide protein complex. Therefore, we believe Anticalin proteins are generally more stable biophysically than antibodies, which are composed of four polypeptide chains. The greater stability and small size of Anticalin proteins as compared to antibodies potentially enable unique routes of Anticalin protein drug administration such as inhaled delivery. Higher-molecular-weight entities, such as antibodies, are often too large to be delivered effectively through these methods. Our Anticalin technology is modular, which allows us to design multimeric Anticalin-based bi- and multi- specific proteins to bind with specificity to two or more disease targets at the same time. This multispecificity offers advantages in biological settings where binding to multiple targets can enhance the ability of a drug to achieve its desired effects, such as facilitating the killing of cancer cells. Moreover, unlike antibodies, the pharmacokinetic, or PK, profile of Anticalin proteins can be adjusted to potentially enable program-specific optimal drug exposure. Such differentiating characteristics suggest that Anticalin proteins have the potential, in certain cases, to become best-in-class drugs.
We have intellectual property rights directed to various aspects of our Anticalin technology platform, allowing for further development and advancement of both our platform and drug candidates. We believe that our ownership or exclusive license of intellectual property related to the Anticalin platform provides us with a strong intellectual property position, particularly in cases where we are seeking to address targets and diseases in a novel way and for which there is existing antibody intellectual property. We also believe that the drug-like properties of the Anticalin drug class have been demonstrated in various clinical trials with different Anticalin-based drug candidates, including PRS-060, PRS-080 and others.
Our core Anticalin technology and platform were developed in Germany, and we have collaborations with major multi-national pharmaceutical companies. We entered into a license and collaboration agreement, or the Servier Collaboration Agreement, with Les Laboratoires Servier and Institut de Recherches Internationales Servier, or Servier, in January 2017 in IO. In May 2017, we entered an alliance with AstraZeneca AB, or AstraZeneca, to treat respiratory diseases, and in February 2018, we entered into a license and collaboration agreement, or the Seattle Genetics Collaboration Agreement, with Seattle Genetics Inc., or Seattle Genetics, in IO.
In connection with our efforts to develop multispecific Anticalin-based proteins designed to engage immunomodulatory targets, we have gained non-exclusive access to antibody building blocks that can be utilized to develop multispecific antibody-Anticalin fusion proteins.
Our current development plans focus on two core pillars, respiratory diseases and IO.
The lead respiratory Anticalin-based drug candidate, PRS-060, binds to IL-4Rα, thereby inhibiting the actions of IL-4 and IL-13, two cytokines (small proteins mediating signaling between cells within the human body) known to be key mediators in the inflammatory cascade that drive the pathogenesis of asthma and other inflammatory diseases. We believe that the small size and biophysical stability of PRS-060 facilitates direct delivery to the lungs through the use of an inhaler, which may enable high pulmonary concentrations of the drug candidate to be achieved at lower doses than would be reached with antibodies that are systemically delivered. Further, we believe an inhaled drug may be better tolerated than systemically-administered antibodies. We completed a phase 1 single ascending dose, or SAD, study of PRS-060 and reported in November 2018 that PRS-060 was safe and well-tolerated by healthy volunteers participating in the trial. We have also initiated and continue to enroll individuals with mild asthma in a multiple ascending dose, or MAD, phase 1 study. This study will evaluate the safety, tolerability and fractional exhaled nitric oxide, or FeNO, reducing potential of PRS-060 versus placebo. Along with our partner AstraZeneca, we anticipate reporting the data from both phase 1 studies at future medical meetings.
We are sponsoring the phase 1 study for PRS-060, after which AstraZeneca will be responsible for further clinical development of PRS-060. We have the right to opt-into further co-development of PRS-060 with AstraZeneca after completion of the phase 2a study. We also have a separate option to co-commercialize PRS-060 with AstraZeneca in the United States. Beyond PRS-060, our alliance includes four additional Anticalin-based drug candidates for treatment of respiratory diseases and two new programs were initiated in 2018 as part of the collaboration. In addition, over the past year, we have initiated two new respiratory programs as part of our proprietary pipeline.
The lead IO Anticalin-based drug candidate in our pipeline, PRS-343, is designed to target the immune receptor 4-1BB and the tumor target HER2. PRS-343 is a genetic fusion of a variant of a HER2-targeting antibody with an Anticalin protein specific for 4-1BB. The proposed mode of action of this 4-1BB/HER2 bispecific is to promote 4-1BB clustering by bridging 4-1BB-positive T cells with HER2-positive tumor cells, thereby providing a potent costimulatory signal to tumor antigen-specific T cells. PRS-343 is intended to localize 4-1BB activation in the tumor, and to thereby both increase efficacy and reduce systemic toxicity compared to 4-1BB-targeting antibodies. Patient dosing in a multicenter, open-label, phase 1 dose escalation study commenced in September 2017. The study is designed to determine the safety, tolerability, and potential anti-cancer activity of PRS-343 in patients with advanced or metastatic HER2-positive solid tumors for which standard treatment options are not available, are no longer effective, or are not tolerated, or in patients that have refused standard therapy. Elevated HER2 expression is associated with multiple cancers, including gastroesophageal, bladder, breast, and a range of other tumor types. We continue to enroll and treat patients in this phase 1 dose-escalation study and intends to report comprehensive data from the study in 2019. We also continue to enroll patients in a dose escalation study of PRS-343 in combination with atezolizumab and intend to report data from this study in 2019.
In January 2017, we initiated a strategic collaboration with Servier to discover and develop five Anticalin-based bispecific therapeutics in IO. The lead program in the alliance is PRS-344, a PD-L1/4-1BB antibody-Anticalin bispecific, currently in investigational new drug application, or IND, -enabling studies. Preclinical data for the PRS-344 program were presented at the Society for Immunotherapy of Cancer, or the SITC, 2018 Annual Meeting. We have achieved two preclinical milestones under the program, one in December 2018 and another in February 2019, and intend to file an IND for the drug candidate in the second half of 2019. We also executed our option to opt-into co-development and US commercialization of PRS-344 during the first quarter of 2019.
In February 2018, we initiated a strategic collaboration with Seattle Genetics to discover and develop up to three Anticalin-based tumor-targeted bispecific therapeutics in IO. As part of the alliance, we have generated and characterized the first tumor-targeting bispecific for further evaluation and development by Seattle Genetics.
We continue to explore opportunities to develop additional differentiated Anticalin-based multispecific therapeutics in IO. We are performing proof of concept and proof of mechanism studies on additional fully proprietary programs to support drug candidate nomination.
The third clinical-stage Anticalin drug candidate, PRS-080, is a polyethylene glycol, or PEG, conjugated Anticalin protein that binds to hepcidin, a natural regulator of iron levels in the blood. An excess amount of hepcidin can cause functional iron deficiency, or FID, which often cannot be treated adequately with iron supplements and can lead to anemia. PRS-080 is designed to target hepcidin for the treatment of FID in anemic patients with chronic kidney disease, or CKD, particularly in
end-stage renal disease, or ESRD, patients requiring dialysis. We believe that by blocking the actions of hepcidin, PRS-080 may serve to address anemia by mobilizing iron from the endogenous iron stores in the body for incorporation into red blood cells. With a serum half-life of several days, PRS-080 was designed to inhibit hepcidin sufficiently to mobilize functional serum iron for erythropoiesis, followed by recovery of blood hepcidin levels to prevent iron overload.
PRS-080 has been investigated in SAD phase 1a and 1b studies, first in healthy subjects (1a), then in stage 5 CKD patients requiring hemodialysis (1b), as well as in a multidose phase 2a study in anemic stage 5 CKD patients requiring hemodialysis. In these studies, intravenous PRS-080 administrations were safe and well tolerated up to the tested dose of 16 mg/kg in healthy volunteers and up to the tested dose of 8 mg/kg in end-stage CKD patients. The phase 1a and 1b studies were completed in 2015 and 2017, respectively. Based on the phase 1 study results, a multicenter, randomized, double-blind, placebo-controlled, MAD (two cohorts of 4mg/kg and 8 mg/kg, respectively) pilot phase 2a study in anemic hemodialysis dependent CKD patients commenced in the third quarter of 2017. This study was designed primarily to obtain initial results on the safety, tolerability, and pharmacological activity of 5 once weekly doses of PRS-080, and secondarily to evaluate the effect of repeated PRS-080 administration on hemoglobin levels in this patient population. We completed dosing all patients in the phase 2a study in 2018. We intend to present the full data set from this study in 2019. In February 2017, we signed an exclusive option agreement, or the ASKA Option Agreement, with ASKA Pharmaceutical Co., Ltd., or ASKA, granting them an exclusive option to develop and commercialize PRS-080 in Japan, South Korea and certain other Asian markets (excluding China). We also plan to share the phase 2a data with ASKA, at which point ASKA will decide whether to exercise its option to develop and commercialize PRS-080 in Japan and other Asian territories. Additionally, we plan to share the dataset with others for potential partnerships outside of the ASKA territories.
Strategy
Our goal is to become a fully-integrated biotechnology company by discovering and developing Anticalin-based therapeutics to target validated disease pathways in unique and transformative ways, and to later commercialize our therapeutic products. We intend to engage with partners for many of our programs in a combination of geographic and indication-based arrangements to maximize our business opportunities. We also intend to retain certain development and commercial rights on selected products as our experience in drug development grows. Key elements of our strategy include:
| |
• | Completing PRS-060 phase 1 studies. Our phase 1 SAD study for PRS-060 has been completed, and we initiated a MAD study for PRS-060 in the second half of 2018. This study will evaluate the safety, tolerability and FeNO-reducing potential of PRS-060 versus placebo. Along with our partner AstraZeneca, we anticipate reporting the data from both phase 1 studies at future medical meetings. |
| |
• | Advancing PRS-343 through phase 1 dose escalation first-in-patient study followed by expansion studies and combination regimens in selected HER2 positive tumor patient populations with major unmet needs. We initiated a multicenter phase 1 study with PRS-343 in September 2017, which is ongoing. The study aims to assess safety and tolerability of PRS-343 across a range of HER2-positive tumor types. In addition, a multicenter phase 1 study with PRS-343 in combination with atezolizumab commenced in August 2018, which is also ongoing. The study aims to assess safety and tolerability of PRS-343 in combination with the PD-L1 inhibitor atezolizumab across a range of HER2-positive tumor types. We intend to report comprehensive data from monotherapy study, as well as data from the atezolizumab combination study, in 2019. |
| |
• | Advancing PRS-344 to initiation of phase 1 studies. PRS-344 is currently undergoing IND-enabling activities and we intend to file an IND for the program later in 2019. |
| |
• | Reporting PRS-080 phase 2a study data and pursuing partners who will continue development of the drug candidate. In the fourth quarter of 2018, we dosed the final patient in a phase 2a study of PRS-080 in anemic, hemodialysis-dependent CKD patients. This study is intended primarily to obtain initial results on the safety, tolerability, and pharmacological activity of 5 once-weekly doses of PRS-080 and, secondarily, to evaluate the effect of repeated PRS-080 administration on hemoglobin levels in this patient population. We intend to present the full data set from this study in 2019. We also plan to share these data with ASKA, at which point ASKA will decide whether to exercise its option to develop and commercialize PRS-080 in Japan and other Asian territories. Additionally, we plan to share the dataset with others for potential partnerships outside of the ASKA territories. |
| |
• | Continuing to build our platform by entering into new partnerships and license and collaborative arrangements and advancing our currently partnered programs. We have entered into partnership and collaborative arrangements with pharmaceutical companies in a diverse range of therapeutic areas and geographies. We have active strategic partnerships with the global pharmaceutical companies Servier, AstraZeneca and Seattle Genetics. Together with our |
partners, we intend to advance multiple drug candidates through preclinical studies and to select further drug candidates for clinical development in the future. We will also continue to seek to engage with new pharmaceutical partners that can contribute funding, experience and marketing ability for the successful development and commercialization of our current and future drug candidates.
| |
• | Pursuing additional opportunities for our Anticalin technology. We intend to continue to identify, vet and pursue opportunities to develop novel Anticalin therapeutics for respiratory diseases, oncology and additional diseases. |
Anticalin Platform Technology
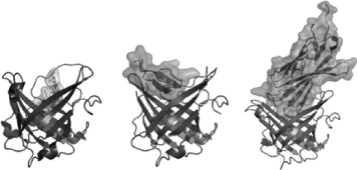
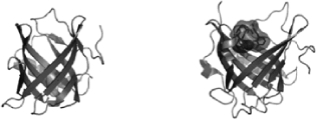
Our platform technology focuses on low molecular-weight Anticalin proteins that bind tightly and specifically to a diverse range of targets. Anticalin proteins are derived from human proteins called lipocalins, which are naturally occurring low-molecular weight human proteins of approximately 17 to 21 kDa molecular mass typically found in blood plasma and other bodily fluids. The lipocalin class of proteins defines a group of extracellular specific-binding proteins that, collectively, exhibit extremely high structural homology, yet have a low amino acid sequence identity (less than 20%), making them attractive “templates” for amino acid diversification. Lipocalins naturally bind to, store and transport a wide spectrum of molecules. The defining attributes of the 12-member human lipocalin class and, by extension, Anticalin proteins, engineered from the lipocalin class of proteins, are a rigidly conserved beta-barrel backbone with four flexible loops regions, which, together, form a cup-like binding pocket. The graphic below shows both tear lipocalin (left) and neutrophil gelatinase-associated lipocalin (NGAL, right).
We currently develop our Anticalin proteins from either tear lipocalin, found primarily in human tear fluid as well as the lung epithelium, or NGAL, a protein involved in the innate immune system, by making discreet mutations in the genetic code for the binding regions. These mutations have the potential to lead to highly specific, high-affinity binding for both small and large molecular targets. Mutations are introduced at pre-defined positions, creating exponentially diverse pools of Anticalin proteins, the most potent and well behaved of which are selected and optimized in a customized manner through in vitro selection using techniques such as phage display, a successful technique in antibody-based drug discovery. The ability to generate highly-diverse and high-quality Anticalin libraries and select for the best binders among the large pool of Anticalin proteins by phage display technology gives us the opportunity to select specific and high affine Anticalin proteins for a wide variety of targets. The flexibility inherent in the Anticalin proteins’ cup-like structure allows us to choose both small-molecule targets that can be bound more inside the ‘cup’ as well as larger protein targets that can be bound more by the flexible loop region outside of the 'cup'. Our phase 1a study for PRS-080, our prior phase 1 study of PRS-050, as well as Daiichi's phase 1 study of a PCSK9-specific Anticalin protein, indicate that these proteins may be non-immunogenic and thereby have the potential to exhibit a favorable safety profile.
The below graphic illustrates Anticalin proteins binding to a small molecule (left), a small protein target (hepcidin, center) and a large protein target (CTLA4, right):
To obtain a specific Anticalin protein, we take advantage of the breadth of our proprietary Anticalin libraries, generated through our protein engineering expertise. We have created, and will continue to create, proprietary Anticalin libraries by rationally diversifying the lipocalin regions, thereby generating Anticalin libraries suitable for identifying binders to different types of targets. By utilizing bacterial production from the earliest stages of drug discovery through Current Good Manufacturing Practice, or cGMP, manufacturing, we have created a seamless platform that improves the quality, yield and cost-effectiveness
of our drug candidates comprising of a single Anticalin protein. Anticalin-based bi- and multi- specific drug candidates, such as PRS-343 and PRS-344 are expressed in standard mammalian expression systems. In this way, Anticalin protein manufacturing is not limited to bacterial systems, with the underlying expression system being driven on a program-by-program basis. See “—Manufacturing” below.
Anticalin proteins share many of the favorable qualities of antibodies, including:
| |
• | High specificity to their targets. Like antibodies, Anticalin proteins can bind their targets without binding other molecules, even molecules with very similar chemical structures or amino acid sequences, allowing for more effective treatments through, for example, minimizing off-target effects. |
| |
• | Tight binding and effective biological activity at their targets. Like antibodies, Anticalin proteins are able to bind their targets at subnanomolar affinities. Anticalin proteins can potentially achieve desirable biological effects by inhibiting an undesired or inducing a desired cell activity by binding to cell-surface receptors or their ligands. |
| |
• | Scalability for large-scale production. Like antibodies, Anticalin proteins lend themselves to large-scale production, yet can also be produced in a range of expression systems ranging from prokaryotic (bacterial) to eukaryotic (for example, animal and fungal) cells. Anticalin proteins can take advantage of several well-understood and widely-practiced methods of protein production both in small amounts for preclinical testing and at larger scale for clinical trials and commercial production. |
While often compared to antibodies, we believe Anticalin proteins offer several advantages over antibodies, including:
| |
• | Small size and biophysical stability. Anticalin proteins are small in size and are monomeric. Therefore, we believe Anticalin proteins are generally more stable biophysically than antibodies composed of four polypeptide chains, which will potentially enable unique routes of administration, such as pulmonary delivery. Higher-molecular-weight entities such as antibodies are often too large to be formulated and delivered effectively through these methods. We believe Anticalin proteins will also be less expensive to manufacture than antibodies due to their lower molecular weight and less bulky structure as well as the ability to leverage the prokaryotic-based manufacturing systems, a less costly manufacturing system than mammalian cell-based manufacturing systems, to create them. |
| |
• | Optimization of half-life. Anticalin proteins can be engineered to have a half-life that is optimal for the indication area and a desired dosing schedule. Antibodies typically have half-lives of two weeks or longer, whereas Anticalin proteins can be engineered to have half-lives from hours to weeks, depending on the half-life extension technology employed, if any. This optionality allows us to exert greater control over the amount of circulating Anticalin protein in the blood and the amount of time such Anticalin proteins circulate in the blood, depending on the underlying biology we are trying to address. |
| |
• | Platform for higher-order multispecificity and avoidance of cross-linking. Our Anticalin technology allows for monovalent or multivalent target engagement, including multispecificity within a single protein. We believe that a monovalent “backbone” is an advantage in situations where pure antagonism of certain cellular receptors is desired. The dual-binding nature of antibodies, which have two “arms,” can be a disadvantage in cases when the antibodies bind to and cross-link cell-surface receptors. Such cross-linking often leads to undesirable activation of the cells bearing those receptors. Single-action (monovalent) Anticalin proteins have only a single binding site and by that do not induce cross-linking. Further, when it is called for by the biology we are addressing, we can create multispecific Anticalin proteins that can simultaneously bind (i) two or more different targets or (ii) different epitopes on the same target by genetically linking Anticalin proteins with distinct specificities or by genetic fusion of an Anticalin protein with an antibody. We believe this multispecificity offers advantages in biological settings where binding to multiple targets can enhance the ability of a drug to achieve its desired effects, such as killing cancer cells. Unique Anticalin proteins can be expressed together and undergo simultaneous target engagement as a single fusion protein, without generally compromising on manufacturability. |
| |
• | Flexible formatting facilitates selection of potent T-cell engagers. The molecular architecture of Anticalin proteins as a single polypeptide chain that folds into a stable eight-stranded β-barrel with exposed N- and C-termini, both not part of the binding site, makes them ideal building blocks to generate bispecific and even multispecific fusion proteins offering novel therapeutic modalities. Multispecific Anticalin-based fusion proteins can be used to pursue innovative therapeutic strategies in IO, particularly by addressing the “immunological synapse” that forms at the interface upon contact between an immune cell and a cancer cell. This can drive an efficient activation of tumor-specific T cells in the vicinity of the tumor, thereby avoiding some of the toxicities observed with peripheral T-cell activation in healthy |
tissues. Generally, the formatting flexibility of Anticalin-based biologics offers the ability of modulating valency and geometry of the multispecific compound according to biological needs. For example, Anticalin proteins can be genetically fused to either the N- or C- terminus of the antibody heavy or light chain, thereby resulting in different geometries of the fusion protein with the antibody as well as Anticalin binding sites covering a range of distances with regard to the T cell target on the one hand and the tumor antigen on the other.
Implementation of the Anticalin Platform Technology: Our Drug Candidate Pipeline
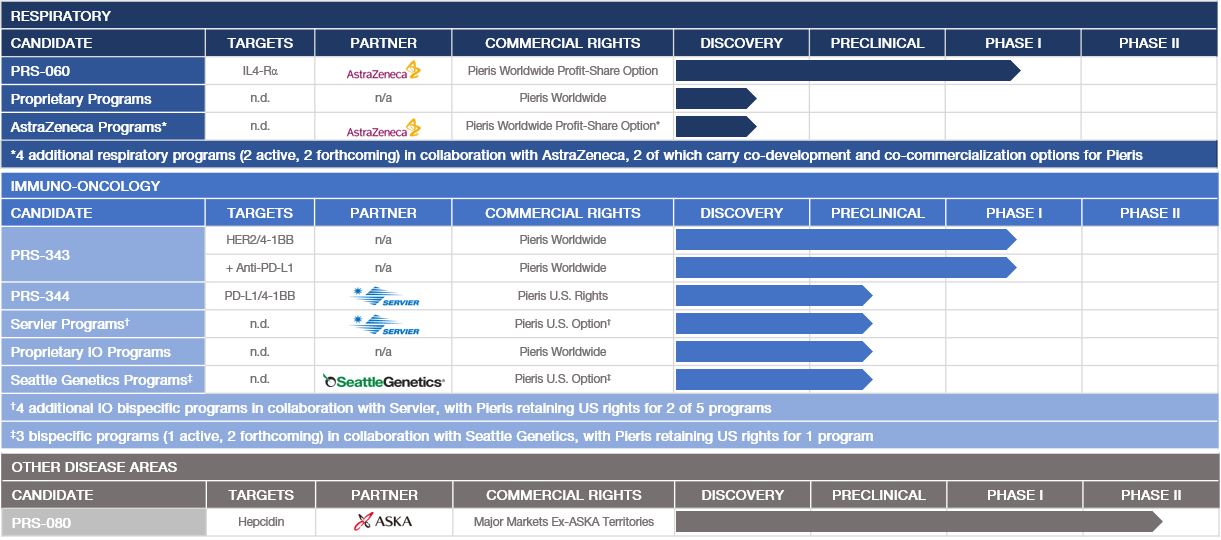
Each of our drug candidates is in the early stage of development, and we anticipate that it will likely be several years before any of our drug candidates could be commercialized. The following table summarizes the status of our current drug candidates and programs:
PRS-060 Targeting IL-4Rα in Asthma
PRS-060 is an Anticalin drug candidate targeting IL-4R, a cell surface receptor expressed on immune cells in the lung. IL-4Rα is specific for the cytokine IL-4 and the closely related cytokine IL-13, both key drivers of the immune system. PRS-060 is derived from human tear lipocalin, has a 20 pM affinity for human IL-4Rα and has a favorable stability profile. Our data showed in vitro that PRS-060 can inhibit the activity of both IL-4 and IL-13. We completed a phase 1 SAD study in healthy volunteers that demonstrated the drug is safe and well tolerated. We initiated a multi-ascending dose phase 1 study in mild asthmatics during the third quarter of 2018. Although the study is primarily designed to establish safety and tolerability, we will also evaluate the drug's potential to reduce FeNO, an established marker of lung airway inflammation. We believe that PRS-060 represents a first-in-class inhaled biologic targeting IL-4Rα for the treatment of asthma. PRS-060 is being developed in partnership with AstraZeneca, as further described below.
Asthma market
Asthma is a very common chronic airway disorder affecting approximately 300 million people worldwide according to the Global Initiative for Asthma and approximately 26 million Americans according to the US Centers for Disease Control. Of these 26 million, approximately 7 million are children. Asthma is responsible for 13 million physician visits per year including approximately 2 million emergency visits in the United States, according to the American Lung Association. In the United States between 2008 and 2013, asthma was responsible for approximately $3 billion in losses due to missed work and school days, approximately $29 billion due to asthma-related deaths, and approximately $50 billion in medical costs. This resulted in a total cost of asthma in the United States of approximately $82 billion in 2013 (Nurmagambetov, Kuwahara and Garbe, Annals of the American Thoracic Society, http://www.atsjournals.org/doi/pdf/10.1513/AnnalsATS.201703-259OC, Volume 15, No. 3, pp 348-356, March 2018).
In 2016, of the approximately 19 million asthma patients over 12 years of age in the United States, about 41%, or 7.8 million, had moderate-to-severe asthma; of the approximately 47.8 million asthma patients over 12 years of age in Europe, about 45%, or 21.5 million, had moderate-to-severe asthma. About 40% of moderate-to-severe asthma patients have uncontrolled asthma, which amounts to approximately 3.1 million patients with moderate-to-severe uncontrolled asthma in the United States and
approximately 8.6 million in Europe (Artisan Healthcare Consulting analysis, including the following: CDC, Eurostat, Rabe (2004), Cazzoletti (2007), Colice (2012), Hekking (2015)). There are several biologics approved for moderate-to-severe uncontrolled asthma in the United States and Europe. Omalizumab is an anti-IgE monoclonal antibody marketed by Roche/Genentech for moderate-to-severe persistent allergic asthma and chronic idiopathic urticaria; in 2018, Roche/Genentech reported total global sales for omalizumab in the amount of CHF 1,912 million ($1,905 million). Mepolizumab is an anti-IL5 monoclonal antibody marketed by GlaxoSmithKline, or GSK, for severe eosinophilic asthma; in 2018, GSK reported global sales for mepolizumab in the amount of £563 million ($727 million). Benralizumab is an anti-IL5 receptor monoclonal antibody marketed by AstraZeneca for severe eosinophilic asthma; in 2018 AstraZeneca reported global sales for benralizumab in the amount of $297 million. Dupilumab is an anti-IL4Rα monoclonal antibody marketed by Sanofi/Regeneron for atopic dermatitis and moderate-to-severe uncontrolled asthma; in 2018, Sanofi/Regeneron reported total global sales of dupilumab in the amount of $922 million.
Challenges in using conventional therapy
The current standard of care for persistent, moderate-to-severe allergic asthma is high-dose inhaled corticosteroids or ICS often in combination with inhaled long-acting beta-adrenergic agonists, or LABA. In uncontrolled moderate-to-severe allergic asthma, omalizumab is given to patients in addition to ICS/LABA combinations. Omalizumab was approved for this condition in the United States in 2003. Outside of the United States, omalizumab is approved for severe asthma. Omalizumab works by binding to the immune mediator immunoglobulin E, or IgE, and inhibiting IgE-mediated activation of mast cells and basophils, types of white blood cells. It has also been shown to impact some diseases, such as asthma, which are driven by eosinophils, another important class of immune cells. However, patient response to omalizumab has shown to be inconsistent, as reported in a publication by McNicholl and Heaney in 2008 in the journal Core Evidence, which explained that in only some studies did omalizumab improve lung function. Furthermore, general asthma symptoms are also typically unaffected by omalizumab. Finally, in 2007, the US Food and Drug Administration, or the FDA, issued a black box warning for omalizumab due to reported cases of anaphylaxis, a potentially life-threatening allergic reaction suffered by some patients who had taken the drug.
Beyond omalizumab, there are four approved biologics for the treatment of asthma. Three target the IL-5 pathway and one targets IL-4Rα. GSK's, mepolizumab, which targets IL-5, was approved for severe eosinophilic asthma in adults and children older than 12 in 2015. Teva’s reslizumab, also targeting IL-5, was approved in 2016 and AstraZeneca’s benralizumab, which targets IL-5 receptor alpha, or IL-5Rα, was approved in November 2017.
Dupilumab is an antibody that targets IL-4Rα that is delivered subcutaneously and was approved for the treatment of moderate to severe atopic dermatitis in March 2017. In October 2018, Regeneron and its partner Sanofi announced that the FDA had approved dupilumab as "add-on maintenance therapy in patients with moderate-to-severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid-dependent asthma." In the phase 3 Liberty Asthma Quest study, dupilumab (300mg every 2 weeks) in the pre-specified high eosinophilic group (eosinophil blood count of ≥ 300 cells/microliter) demonstrated a reduction in annualized rate of severe exacerbations by 67.4% and an improvement in forced expiratory volume in one second, or FEV1, by 0.24L. The Liberty Asthma Venture trial evaluated dupilumab in oral glucocorticoid dependent severe asthma patients. In the overall population, the percentage of patients that decreased oral corticosteroid use by 50% or more was 80% in the dupilumab group versus 50% for placebo (or a 60% relative reduction), while decreasing the rate of severe exacerbations by 59% and improving FEV1 by 0.22L versus placebo. In the high eosinophilic group, dupilumab decreased the rate of severe exacerbations by 71% and improved FEV1 by 0.32L versus placebo (Rabe et al., 2018).
Advantages to inhalation as a route of administration for PRS-060
PRS-060 was safe and well-tolerated in SAD phase 1 study. The drug candidate is currently being evaluated in a MAD phase 1 study. We believe that local delivery via inhalation may lead to a better tolerability profile than systemically administered antibodies. Since dosing by inhalation is a common route of administration in asthma patients, it could represent a more convenient dosage regimen for patients than dosing of antibodies by injection.
Preclinical data
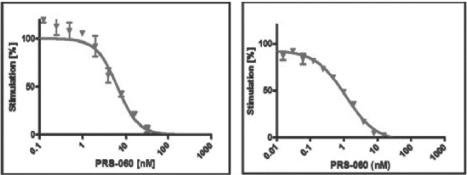
In in vitro assays, PRS-060 specifically bound to immobilized targets such as human IL-4Rα in a concentration-dependent manner. We tested the binding of PRS-060 to various targets in an enzyme-linked immunosorbent assay, or ELISA, a standard in vitro assay platform. In these tests, PRS-060 bound to IL-4Rα with subnanomolar affinity and it did not bind to three other human cell-surface interleukin receptors (IL-6R, IL-18Rα, IL-23Rα). Furthermore, the activity of IL-4 and IL-13 was inhibited
by PRS-060 in a dose-dependent manner. The charts below show the inhibition of IL-4- (left) or IL-13- (right) induced proliferation in human TF-1 cells in vitro by PRS-060.
In in vivo assays in mice genetically altered to express human IL-4Rα, human IL-4 and IL-13, low doses of PRS-060 inhibited the induction of eotaxin protein, a marker of airway inflammation, in lung tissue following pulmonary delivery. We observed this inhibition at both the RNA and protein levels compared both to buffer and to tear lipocalin (control).
The chart below shows the duration of PRS-060-mediated inhibition of eotaxin gene expression, a marker of airway inflammation, in lung tissue by a single pulmonary dose in mice:
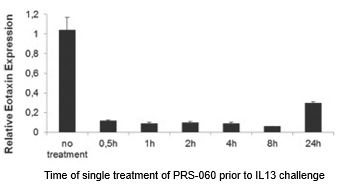
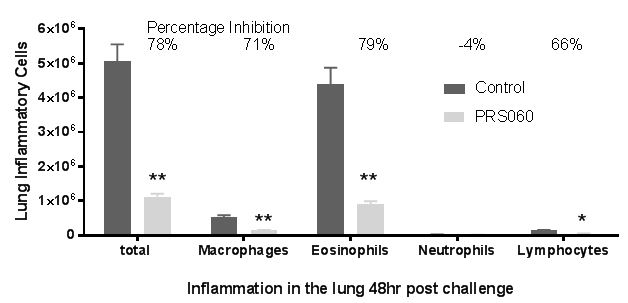
When we administered IL-13 into the lung of humanized mice (that express human IL-4, IL-13 and IL-4Rα), inflammation was induced as determined by eotaxin expression, which was not inhibited when phosphate buffered saline, or PBS, or human wild type lipocalin was administered into the lung. In contrast to the PBS administration, increases in eotaxin expression were prevented when PRS-060 was administered into the lung before IL-13. As demonstrated in the above chart, the model showed the inhibitory potential lasts for up to 24 hours after PRS-060 administration. We have also demonstrated that PRS-060 reduces the inflammation associated with antigen challenge in a mouse asthma model. The chart below shows that pre-treatment with PRS-060 reduces the lung levels of the key inflammatory cells' eosinophils and lymphocytes, a profile that supports the hypothesis that lung delivery of an IL-4Rα antagonist to asthmatics may be viable approach to the treatment of asthma.
Clinical data
In November 2018, we disclosed that PRS-060 was safe and well tolerated in the SAD, healthy volunteer, phase 1 study. A MAD phase 1 study is currently enrolling mild asthmatics with elevated levels of FeNO (>35 ppb), testing the safety and tolerability of PRS-060 administered twice daily for 9 days and once on a final, 10th day. In addition, the MAD study will evaluate the FeNO-lowering potential of PRS-060 versus placebo. We plan to disclose data from both studies at upcoming medical meetings.
Proprietary Respiratory Platform
We are developing a proprietary Anticalin protein pipeline for asthma and other respiratory diseases via inhaled administration. The company initiated two new programs directed to discovering and developing Anticalin proteins for respiratory diseases during 2018.
AstraZeneca Respiratory Collaboration Beyond PRS-060
Our license and collaboration agreement with AstraZeneca, or the AstraZeneca Collaboration Agreement, includes four programs beyond PRS-060. We retain co-development and co-commercialization rights to two out of those four programs. We have initiated discovery work on the first two additional development candidates under the collaboration. The targets and disease areas of those two programs are undisclosed.
PRS-343 Targeting 4-1BB (CD-137) in Oncology
PRS-343 is a bispecific protein targeting the immune receptor 4-1BB and the tumor target HER2. It is generated by genetic fusion of an Anticalin protein specific for 4-1BB to each heavy chain of a variant of a HER2-targeting antibody. The mode of action of this 4-1BB/HER2 bispecific is to promote 4-1BB clustering by bridging 4-1BB-positive T cells with HER2-positive tumor cells, and to thereby provide a potent costimulatory signal to tumor antigen-specific T cells. PRS-343 is intended to localize 4-1BB activation in the tumor, and to thereby both increase efficacy and reduce systemic toxicity compared to 4-1BB-targeting antibodies being developed by third parties in clinical trials. We initiated a phase 1 dose-escalation study of PRS-343 in HER2 positive patients in September 2017 and a phase 1 dose-escalation study of PRS-343 in combination with atezolizumab in HER2 positive patients in August 2018. We intend to report comprehensive data from the monotherapy study, as well as data from the combination study, in 2019.
Biology of the costimulatory immune receptor 4-1BB
4-1BB, is a co-stimulatory immune receptor and a member of the tumor necrosis factor receptor, or TNFR, super-family. It is mainly expressed on activated CD4+ and CD8+ T cells, activated B cells, and natural killer, or NK, cells. 4-1BB plays an important role in the regulation of immune responses and thus is a target for cancer immunotherapy. 4-1BB ligand, or 4-1BBL, is the only known natural ligand of 4-1BB and is constitutively expressed on several types of antigen-presenting cells, or APC. 4-1BB-positive T cells are activated by engaging a 4-1BBL-positive cell. The induced 4-1BB clustering leads to activation of the receptor and downstream signaling. In a T cell pre-stimulated by the T cell receptor, or TCR, binding to a cognate major histocompatibility complex, or MHC, target, costimulation via 4-1BB leads to further enhanced activation, survival and proliferation, as well as the production of pro-inflammatory cytokines and an improved capacity to kill.
Validation of 4-1BB as a therapeutic target in cancer
The benefit of 4-1BB costimulation for the elimination of cancerous tumors has been demonstrated in a number of murine in vivo models. The forced expression of 4-1BBL on a tumor, for example, leads to tumor rejection. Likewise, the forced expression of an anti-4-1BB single chain antibody fragment, or scFv, on a tumor leads to a CD4+ T cell and NK-cell dependent elimination of the tumor. A systemically administered anti-4-1BB antibody has also been demonstrated to lead to retardation of tumor growth.
Human ex vivo data support the potential of 4-1BB as a costimulatory receptor in cancer therapy: It has been reported that for T cells isolated from human tumors, 4-1BB is an effective marker for those that are tumor-reactive. Based on this observation, we believe that anti-4-1BB antibodies can be utilized to improve adoptive T cell therapy, or ACT, by augmenting the expansion and activity of CD8+ melanoma tumor-infiltrating lymphocytes, or TILs.
Finally, the potential of 4-1BB targeting has also been shown in nonclinical combination therapy studies, where an additional benefit was demonstrated by combination of 4-1BB agonism with checkpoint blockade or NK cell-targeting antibodies.
Current approaches to clinical 4-1BB targeting
The demonstration of the potential therapeutic benefit of 4-1BB costimulation in nonclinical models has spurred the development of therapeutic antibodies targeting 4-1BB, utomilumab and urelumab.
Utomilumab is a fully humanized IgG2 antibody that binds 4-1BB in a manner that blocks the binding of endogenous 4-1BBL to 4-1BB, and that according to publicly available data is well tolerated as a monotherapy and in combination with rituximab.
Urelumab is an IgG4 antibody that, in contrast to utomilumab, binds 4-1BB in a manner that does not interfere with the 4-1BB / 4-1BBL interaction. While an initial study reported manageable toxicity with doses up to 10mg/kg, a follow-up monotherapy phase 2 study was reported to have been stopped due to an “unusually high incidence of grade 4 hepatitis.” Prior clinical trials with urelumab were focused on safety and efficacy at lower doses as monotherapy or in combination, for example, with rituximab (NCT01775631).
Rationale for bispecific targeting of 4-1BB
We believe that the natural mode of activation of 4-1BB, which requires receptor clustering, demonstrates that an ideal 4-1BB-targeting agent should firstly lead to clustering of 4-1BB, and secondly do so in a tumor-localized fashion on TILs. The antibodies currently in clinical development are not ideal in that respect, as 4-1BB clustering can only be induced by binding to Fcg receptor-positive cells, which are not selectively tumor-localized but distributed throughout the body for Fcg-dependence of TNFR targeting. The toxicity data of urelumab indicates that such a non-selective activation leads to unacceptable toxicity, potentially making it impossible to find a therapeutic window for such 4-1BB-targeting antibodies.
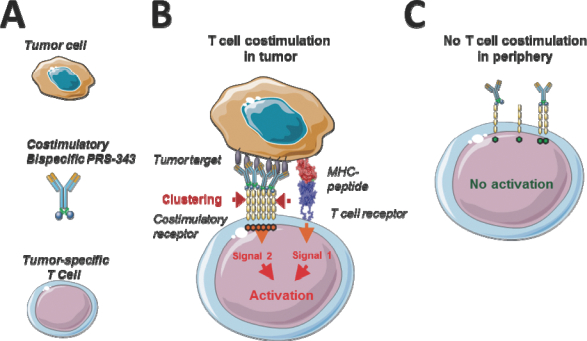
We therefore hypothesized that to obtain an ideal 4-1BB-targeting agent, a bispecific molecule should be designed that targets 4-1BB on one end and a differentially expressed tumor target on the other end. A visualization of the general concept is provided in Figure 1, below. HER2/4-1BB bispecific is envisioned to promote 4-1BB clustering by bridging T cells with HER2-positive tumor cells, and to thereby provide a potent costimulatory signal to tumor antigen-specific T cells, further enhancing its TCR-mediated activity and leading to tumor destruction.
|
| |
Figure 1 | Concept of costimulatory T cell engagement. (A) The elements of the system are a target-positive tumor cell, a T cell with a TCR that is specific for an HLA/peptide combination on the tumor, and a costimulatory bispecific. (B) Within a patient´s tumor, tumor-specific T cells are bridged with tumor cells by a costimulatory bispecific. The resulting clustering of the costimulatory TCR provides a local co-activating signal to the T cell, further enhancing its TCR-mediated activity and leading to tumor destruction. (C) Toxic side effects are expected to be manageable, as target-negative cells do not lead to costimulation of T cells due to a lack of target-mediated receptor clustering, and healthy tissue is spared by tumor-costimulated T cells due to the absence of a primary, TCR-mediated signal. Design and Generation of HER2/4-1BB bispecific PRS-343. |
To obtain a molecule that would work by the mode of action of costimulatory T-cell engagement, we generated the HER2/4-1BB bispecific PRS-343. The molecule consists of two different building blocks binding to the two targets HER2 and 4-1BB. To generate the 4-1BB-specific building block of PRS-343, we utilized Anticalin technology. A 4-1BB-binding Anticalin protein was generated based on a re-design of the natural binding pocket of NGAL using mutant Anticalin libraries and a selection and screening process. The lead 4-1BB-binding Anticalin protein binds human 4-1BB with an affinity of 2 nM as determined by surface plasmon resonance, or SPR, and is capable of costimulating human T cells when immobilized on a plastic dish together with an anti-CD3 antibody.
To generate the bivalent HER2/4-1BB bispecific PRS-343, we constructed a genetic fusion of a 4-1BB-specific Anticalin protein to the C-terminus of each heavy chain of the trastuzumab IgG4 variant, connected by a flexible, non-immunogenic 15 amino acid linker sequence.
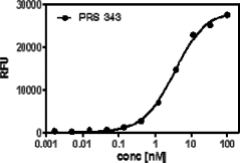
We utilized a Sandwich ELISA experiment to investigate whether PRS-343 can bind both targets at the same time, which is a necessary prerequisite for the envisioned mode of action of PRS-343. Figure below shows that a sigmoid binding curve results from this titration, proving that both targets can indeed be engaged at the same time, fulfilling the key requirement for simultaneous costimulatory engagement of T cells by HER2-positive target cells.
|
| |
Figure 2 | PRS-343 simultaneous binding to targets HER2 and 4-1BB. Recombinant Her2 was coated on a microtiter plate, followed by titration of PRS-343. Subsequently, a constant concentration of biotinylated human 4-1BB was added, which was detected via a peroxidase-conjugated avidin variant. |
Mode of action – costimulatory T cell activation
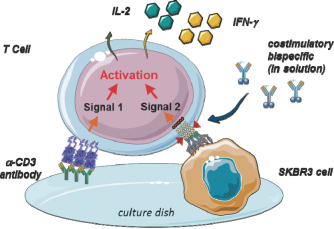
We developed a novel T cell activation assay format to investigate whether PRS-343 is capable of costimulating T cells that have received a basic stimulus via the TCR. The assay, visualized in Figure 3 below, is based upon providing the TCR stimulus via an anti-CD3 antibody coated onto the plastic culture dish, while 4-1BB costimulation is achieved by tumor-target dependent clustering of 4-1BB on purified T cells.
|
| |
Figure 3 | Visualization of costimulatory T cell activation assay. HER2-positive tumor cells are grown overnight on cell culture plates that have been precoated with low amounts of an anti-CD3 antibody to provide a limited primary activation of T cells via the T cell receptor. T cells are added to the wells together with the titrated 4-1BB/HER2 bispecific PRS-343, leading to clustering of the costimulatory 4-1BB receptor, which in turn results in T-cell costimulation. T cell costimulation is detected by increased supernatant IL-2 and IFN-γ levels in the culture supernatants after continued culture. |
There is a clear induction of IL-2 (Figure A) and IFN-γ (Figure C) with increasing concentrations of PRS-343. The fitted EC50 of this effect is similar for both proinflammatory cytokines, with 0.7 nM for IL-2 induction and 0.3 nM for IFN-α induction, respectively. That T-cell costimulation is indeed, due to the bispecific engagement of T cells and SKBR3 cells, shown by two observations: firstly, the monospecific antibody trastuzumab does not lead to enhanced T cell activation (average shown as dotted line in Figure A and Figure C), and secondly, disrupting the bispecific interaction with an excess of trastuzumab abolishes the effect of IL-2 and INF-γ induction almost completely, except at the highest concentrations of PRS-343 employed (Figure B and Figure D).
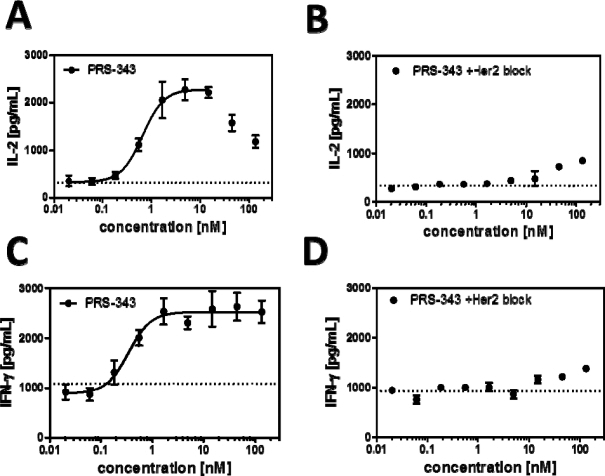
|
| |
Figure 4 | Experimental result of costimulatory T cell activation assay. HER2-positive SKBR3 tumor cells were grown overnight on 96-well plates that had been precoated with 0.25 µg/mL anti-CD3 antibody for 1 h at 37°C. The next day, T cells purified from healthy donor PBMC were added to the wells together with the titrated 4-1BB/HER2 bispecific PRS-343 (filled circle) or trastuzumab as a control (dotted line). After three days in culture, IL-2 (A) and IFN-γ, levels in the culture supernatants were measured by an electrochemoluminescence immunoassay. In parallel, the experiment was performed in the presence of an excess of trastuzumab (340 nM) to inhibit the binding of PRS-343 to the SKBR3 cells, and IL-2 (C) and IFN-γ (D) levels were measured. |
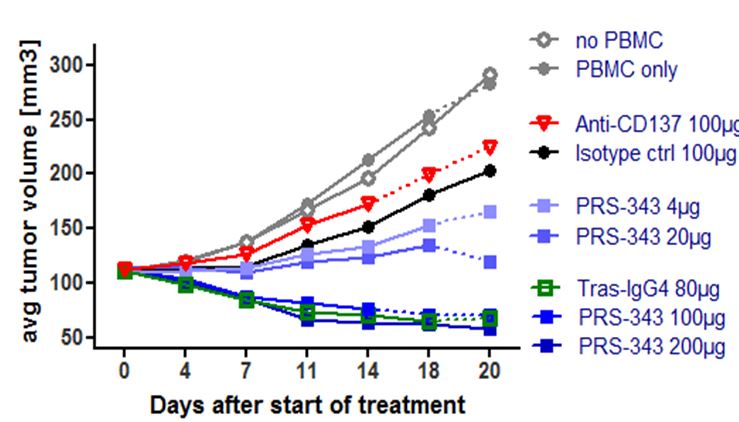
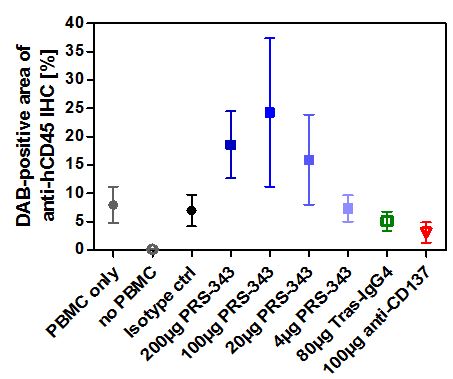
Proof of concept data utilizing a humanized SK-OV-3 mouse model demonstrated dose-dependent tumor growth inhibition compared to treatment with the isotype control (Figure 5). It is anticipated that the tumor growth inhibition, or TGI, in this model is predominantly caused by the anti-HER2. The anti-tumor response observed with PRS-343 was accompanied by a significantly higher tumor infiltration with human lymphocytes (hCD45+). Interestingly, the anti-4-1BB benchmark neither displayed tumor growth inhibition nor enhanced lymphocyte infiltration into tumors compared to isotype. The tras-IgG4 control was also devoid of lymphocyte infiltration into the tumor but displayed a tumor growth inhibition comparable to PRS-343. Taken together, these data show that PRS-343 provided dual activity by both increasing the frequency of TILs by bispecific targeting of CD137 and HER2 as well as mediating direct tumor growth inhibition by the direct, monospecific targeting of HER2.
|
| |
Figure 5 | PRS-343 activity in NOG mice engrafted with HER2-positive SK-OV-3 cell line and human PBMC. (A) Median of tumor growth. (B) Frequency of CD45+ cells determined by immunohistochemistry of tumors after study end. |
PRS-344
PRS-344 consists of a PD-L1 targeting antibody and 4-1BB targeting Anticalin proteins genetically fused to each arm of the C-terminal heavy chain of the antibody. The Anticalin moiety of PRS-344 is a single domain protein based on the extracellular human protein NGAL that has been engineered to bind 4-1BB with high affinity and selectivity.
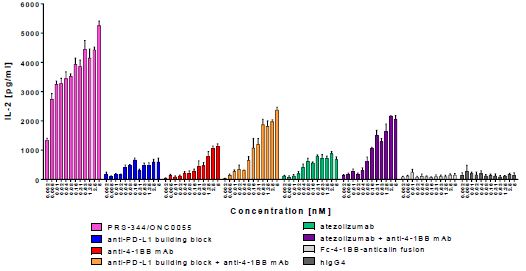
4-1BB is a costimulatory receptor belonging to the TNFR super-family. Clustering of 4-1BB on the surface of T cells leads to T cell activation, proliferation and cytokines secretion. The mode of action of PRS-344 is to promote 4-1BB clustering by bridging 4-1BB-positive T cells with PD-L1-positive tumor cells, and to thereby provide a potent costimulatory signal to tumor antigen-specific T cells. PRS-344 is intended to localize 4-1BB activation in the tumor in a PD-L1 dependent manner. PD-L1 is a transmembrane protein belonging to the B7 family and is expressed on a variety of cells including T cells, B cells, epithelial and vascular endothelial cells. Most importantly, PD-L1 is found at high levels on tumor cells of several cancer types including but not limited to melanoma, lung, bladder, colon, and breast cancer. Binding of PD-L1 to its receptor PD-1 leads to exhaustion of tumor-infiltrating T cells. PRS-344 blocks the PD-1/PD-L1 interaction and thus is capable of reversing T-cell exhaustion in the tumor microenvironment. Preclinical data shows that the synergistic effect observed by targeting PD-L1 and 4-1BB simultaneously is stronger with PRS-344 than with the combination of anti-PD-L1 and anti-4-1BB antibodies.
|
| |
Figure 1 | The combination of atezolizumab and anti-4-1BB benchmark demonstrates the strong synergistic effect of T cell costimulation and checkpoint blockade in T cell activation. With PRS-344, this synergistic effect is massively increased. |
Together with our partner Servier, we plan to initiate a phase 1 study of PRS-344 in the second half of 2019. This first-in-human study will consist in evaluating the safety and tolerability profile of PRS-344 and determining its maximum tolerated dose, or MTD, and/or the recommended phase 2 dose, or RP2D, in patients with solid tumors. In addition, the PK profile as well as pharmacodynamic effects of the PRS-344 will be characterized in the study. Any initial signs of anti-tumoral activity will be correlated to safety and PK and further explored in expansion cohorts.
IO Market with respect to PRS-343 and PRS-344
In 2018 there were approximately 1.735 million estimated new cancer cases in the United States (NCI Surveillance, Epidemiology, and End Results Program) and approximately 18.1 million cancer cases worldwide (IARC GLOBOCAN 2018). The direct medical cost for cancer in the United States in 2015 was estimated to be approximately $80.2 billion by the Agency for Healthcare research and Quality, or the AHRQ.
Checkpoint inhibitors such as PD-1 and CTLA4 have revolutionized the way certain cancers are treated and in 2018 the Noble Prize in Medicine was awarded to Dr. James Allison and Dr. Tasuku Honjo for their discovery of CTLA-4 and PD-1, respectively. By the end of 2018 a total of six anti-PD-1 or PD-L1 monoclonal antibodies and one anti-CTLA4 antibody have been approved in the United States. Global sales in 2018 for these seven checkpoint inhibitors exceeded $16 billion. The majority of the global sales of checkpoint inhibitors comes from two anti-PD-1 monoclonal antibodies: pembrolizumab marketed by Merck & Co and nivolumab marketed by Bristol-Myers Squibb. In 20187, Merck & Co reported sales of $7.171 billion for pembrolizumab and Bristol-Myers Squibb reported $6.735 billion for nivolumab.
Other IO Programs
Current antibody-based therapies targeting tumor cell destruction or immune activation are hampered by, among other factors, low response rates and the induction of immune-related adverse events. Our IO pipeline beyond PRS-343 and PRS-344 is designed to target checkpoint proteins or, like PRS-343, costimulatory proteins. These programs consist of a variety of multifunctional biotherapeutics that can encompass a fusion of antibodies with Anticalin proteins or two or more Anticalin proteins to each other. These combined molecules have the potential to build upon current therapies by modifying or regulating one or more immune functions on a single fusion protein, thereby having the potential to elevate immune responses within a tumor microenvironment. We believe that a tethered Anticalin protein directed at checkpoint or co-stimulatory targets can preferentially activate the immune system at the site of the tumor microenvironment thus providing efficacy with enhanced therapeutic index. We believe that these bispecific constructs represent a “platform within a product” opportunity in IO since it may be possible to apply a single combined Anticalin-antibody molecule in a number of different cancers. This belief is based on the shared underlying biology such as checkpoint and costimulatory biology found within tumors arising in different organs.
Servier Collaboration Beyond PRS-344
Our Servier Collaboration Agreement includes four programs beyond PRS-344. We retain co-development and co-commercialization rights to two out of those four programs. The four additional programs have been defined, which may combine antibodies with one or more Anticalin proteins based on our proprietary platform to generate innovative immuno-oncology bispecific drug candidates.
Seattle Genetics Collaboration
Our collaboration with Seattle Genetics to discover and develop Anticalin-based tumor-targeted bispecific therapeutics in IO includes up to three programs. We retain a co-development and co-commercialization option for one of these three programs.
PRS-080 Targeting Hepcidin in CKD-related FID-anemia
PRS-080 is an Anticalin drug candidate targeting hepcidin, a peptide mediator that is an important negative regulator of iron absorption and storage, derived from the naturally occurring human lipocalin known as NGAL. The normal function of hepcidin is to maintain equilibrium in iron supply for red blood cell production by binding to ferroportin, or FPN, the protein that transports iron from the inside of a cell to the outside, inducing its internalization and subsequent degradation. The binding of hepcidin to FPN reduces the iron uptake from the intestine into the body and inhibits iron mobilization from cellular stores into red blood cells. An excess amount of hepcidin can cause FID, which often cannot be treated adequately with iron supplements and can lead to anemia. According to a 2009 publication by Young and Zaritsky in the Clinical Journal of the American Society of Nephrology, lowering hepcidin levels or antagonizing its actions would reverse the negative effects of inflammation on red blood cell formation by allowing mobilization of stored iron and improved iron absorption.
PRS-080 has been designed to target hepcidin for the treatment of FID in anemic patients with CKD, particularly in ESRD patients requiring dialysis, to allow them to mobilize iron that is trapped in iron storage cells for use in the creation of red blood cells. We have also engineered PRS-080 to have a half-life of less than a week, so that following administration, it is expected to clear from the human body in a much shorter timeframe than antibodies, which typically have a half-life of two weeks or greater. This half-life was achieved by covalently linking PRS-080 to a specific PEG in order to extend the serum half-life of the combined molecule to desirable levels. Since hepcidin is constantly produced by the body, we believe that a frequent, for example, once per week, dosing interval will be optimally suited to interfere with hepcidin function. A shorter half-life than antibodies may be more compatible with this dosing schedule. A longer antibody-like residence time is not seen as advantageous, but rather could lead to the accumulation of both the drug and the target beyond the typical residence time of hepcidin, resulting in large quantities of hepcidin bound to antibodies. We completed a phase 1a SAD study with PRS-080 in healthy volunteers in 2015. Results from this study were presented at the 2015 Annual Conference of the American Society of Hematology (http://www.bloodjournal.org/content/126/23/536). Based on the data obtained, we initiated a phase 1b study in stage 5 CKD patients requiring hemodialysis which we completed in February 2017. We initiated a multi-dose clinical study in CKD patients requiring hemodialysis in the third quarter of 2017, which is assessing the safety and tolerability of multi-dose administration of PRS-080, as well as a secondary assessment of repeated doses of PRS-080 on hemoglobin levels over a period of approximately one month. The final patient was dosed in this phase 2a study in 2018 and we intend to report the results of this study in 2019.
Anemia and FID in the CKD population
Anemia is a serious medical condition in which blood is deficient in red blood cells and hemoglobin, leading to inadequate oxygen delivery to tissues and cells throughout the body. Anemia is generally said to exist when hemoglobin is less than 13 g/dL in men and 12 g/dL in women. Anemia has a number of potential causes, including nutritional deficiencies, iron deficiency, bone marrow disease, medications, and abnormalities in production of or sensitivity to erythropoietin, a hormone that controls red blood cell production. Anemia is a frequent and severe consequence of CKD. In addition, within the CKD population, anemia may be caused by FID. FID exists when, despite adequate stores, iron cannot be mobilized for erythropoiesis. In this case, despite treatment with exogenous erythropoietin and iron supplements, “functional” iron is still deficient. FID-anemic patients can be identified and selected for therapy using marketed laboratory tests for iron metabolism. According to the results of a 2017 research analysis conducted for us by Artisan Healthcare Consulting, there were an estimated 505,000 individuals with ESRD that were on hemodialysis in the US in 2017 and approximately 90% of these patients are treated with erythropoiesis stimulating agents, or ESAs. Up to 50% of the ESRD patients on hemodialysis that are currently treated with ESA have FID. Approximately 15% of the FID patients are still anemic despite ESA treatment. Based on the estimated 505,000 individuals with ESRD on hemodialysis, we believe that approximately 34,000 individuals are FID-anemic despite ESA treatment in the US.
Challenges in using conventional therapy
We believe that CKD patients with FID-anemia are especially poorly served. These patients have adequate stores of iron but this iron is not efficiently incorporated into red blood cell precursors through recombinant erythropoiesis stimulating agents, or rESAs, and iron supplements. According to the 2009 publication by Young and Zaritsky in the Clinical Journal of the American Society of Nephrology, this imbalance in iron metabolism is a result of a high level of circulating hepcidin in the blood stream. We believe that existing therapies are limited in that they do not have an impact on hepcidin or, in the case of rESAs, patients often become resistant to the therapy.
Our potential solution: binding hepcidin with PRS-080
We have engineered PRS-080 so that it binds to hepcidin and reduces the impact of hepcidin’s negative regulation on iron mobilization. We believe that by blocking the actions of hepcidin, PRS-080 may address anemia by mobilizing iron for incorporation into red blood cells.
In patients suffering from anemia of CKD, and specifically in patients with FID, hepcidin is chronically produced by the body in abnormally large amounts. Therefore, we believe that the best way to inhibit its function is to administer an inhibitor on a repeated basis, such as once a week. Our approach will use PRS-080 in connection with a conjugated PEG30 molecule, a well-known half-life extender, in order to allow the drug sufficient residence time in the body. Once coupled to PEG30, PRS-080 is intended to have a half-life that will be optimally suited for dosing anemic patients with CKD. In contrast, antibodies typically have a half-life of two to three weeks. Such a long half-life renders antibodies unsuitable for frequent administration and elimination of a circulating target protein like hepcidin because such antibodies tend to accumulate the target after binding due to their own long residence time in the body with the associated risk of bound hepcidin being released by antibodies that are still circulating in the blood.
Preclinical data
Hepcidin binds to FPN and induces its internalization and subsequent degradation, thus disabling iron mobilization from cells. PRS-080 binds strongly to hepcidin and inhibits its activity in a dose dependent manner as shown in in vitro potency assays. PRS-080 is able to completely inhibit the internalization of FPN above a concentration of 20 nM.
Our preclinical studies targeted the cynomolgus monkey orthologue of hepcidin, which has a high degree of similarity (96% identity) with human hepcidin. PRS-080 was found to bind with high affinity to the cynomolgus monkey version of hepcidin.
We performed a dose finding study in cynomolgus monkeys, testing intravenous 30-minute infusions as well as subcutaneous injections of PRS-080 to study the PK properties of PRS-080 and the functional consequences of hepcidin inhibition on iron mobilization. A dose of 1 mg/kg PRS-080 produced a robust, transient and reversible increase in total iron levels from approximately 36 µM at baseline to 52 µM after 8 hours. Doses higher than 1 mg/kg elevated serum iron concentrations to comparable levels and, in a dose-dependent manner, prolonged the response. A linear correlation was observed over time between the PRS-080 dose increases and the increase of serum iron concentrations. The PK properties of PRS-080 were investigated after a single administration at doses ranging from 20 mg/kg to 150 mg/kg. The concentration over time profiles of PRS-080 showed standard drug-like properties, as the kinetics were dose proportional and there was a low volume of distribution. Elimination of PRS-080 occurred with a terminal half-life of about 2 days, which suggests a 3-day half-life in humans.
We also carried out a 4-week repeated dose toxicology study with intravenous infusions of PRS-080 for 30 minutes every other day. Our work included toxicokinetic and anti-drug antibody, or ADA, measurements. During the study, safety pharmacology parameters on the cardiovascular system and respiration were monitored and all safety endpoints were met. Our preclinical studies also examined a different NGAL-derived Anticalin, or surrogate molecule, which targets rat hepcidin in a rat model of inflammation-induced anemia. In these studies, administration of the surrogate molecule once per day or every other day inhibited the manifestation of anemia in the rats over the course of a three-week period.
The 4-week repeated PRS-080 dosing to cynomolgus monkeys was well tolerated up to the highest tested dose of 120 mg/kg. This dose was classified as producing no AEs as a result of the fact that routine laboratory tests and blood cell examinations did not demonstrate any adverse findings and safety pharmacology investigations were also without AEs. As a result of the hepcidin inhibition, the study showed increased iron uptake and storage, for example in the liver, and mobilization.
The functional consequence of PRS-080 treatment on bone marrow activity and red blood cell production, or hematopoiesis, by means of hemoglobin (an oxygen transporting protein contained in red blood cells) concentration in reticulocytes, a precursor of red blood cells, was investigated in cynomolgus monkeys following repeated administration. As shown in the below chart, after administration of PRS-080 either intravenously (i.v. 150 mg/kg) or subcutaneously (s.c. 20 mg/kg), elevated hemoglobin concentrations in reticulocytes, or Retic CH, were observed on day 30 compared to pre-treatment (pre-dose).
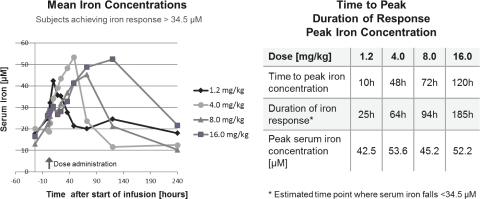
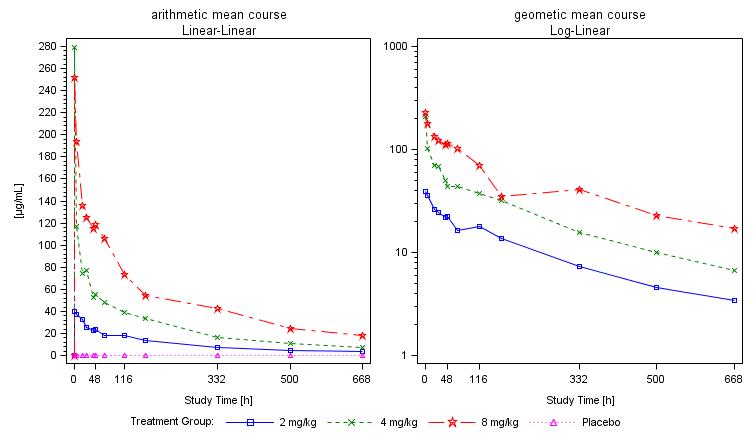
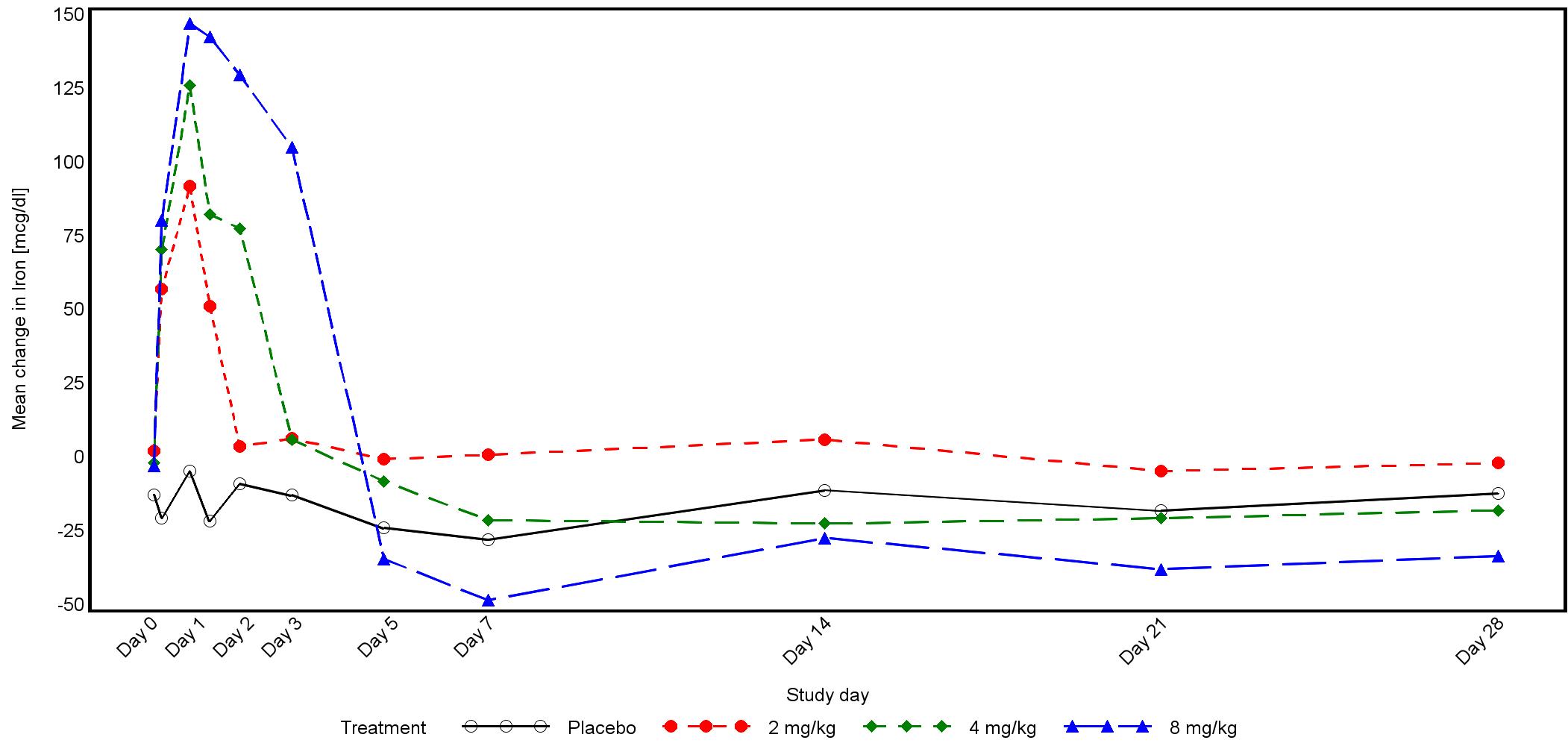
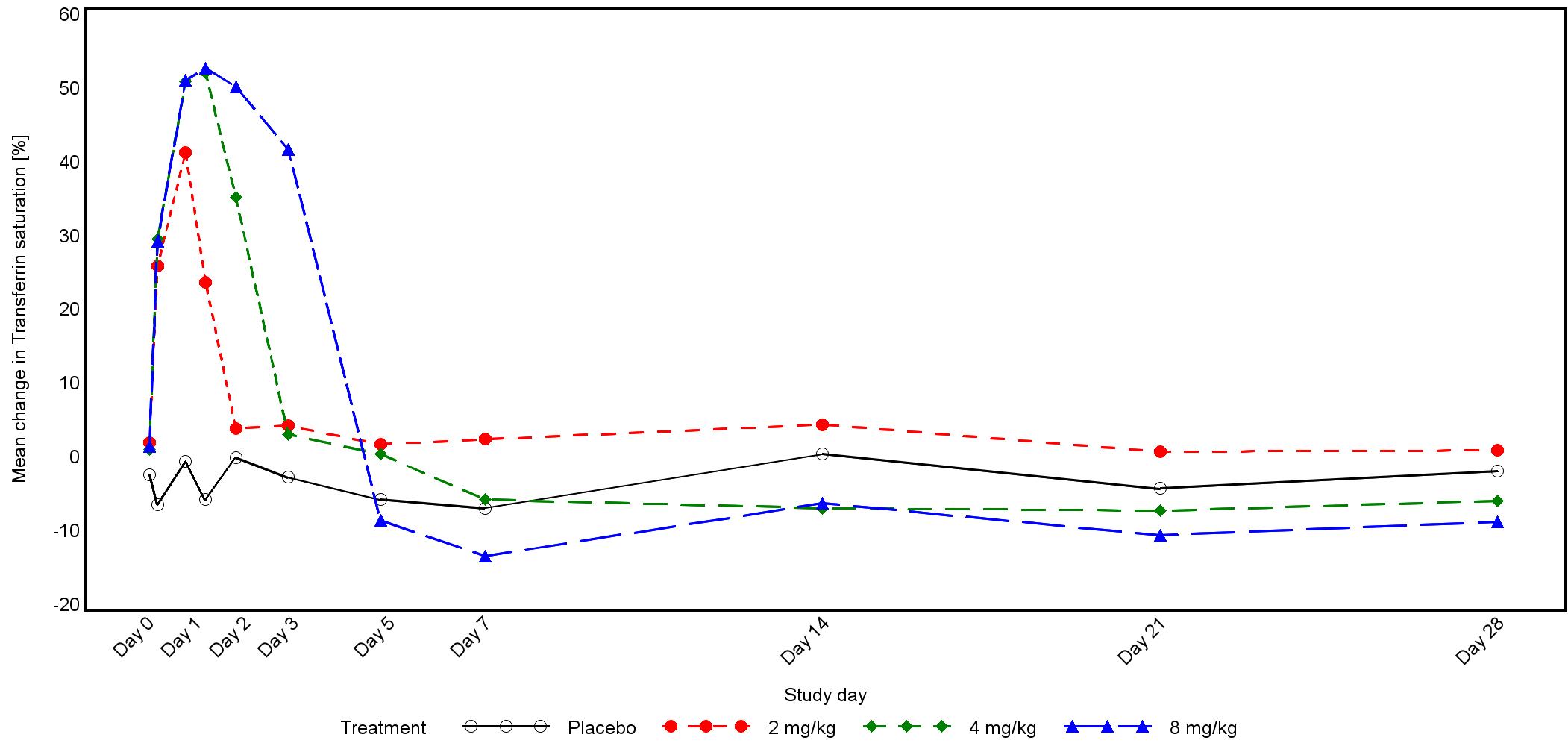
Phase 1 study design and results
The phase 1a study of PRS-080 was conducted in healthy volunteers. The study was a single dose escalating, blinded, placebo-controlled trial at a dose range from 0.2 to 40 mg/kg (equivalent to 0.08 to 16.0 mg/kg based on protein content). Forty-eight subjects were dosed with PRS-080 or a placebo. This study was governed and approved by the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, or the BfArM) and the local ethics committee. Treatment of subjects began at the end of 2014 and was completed in June 2015, followed by evaluation of the data.
PRS-080 was well tolerated. All treatment emergent adverse events, or AEs, were either mild or moderate and no serious adverse events, or SAEs, were observed. No association of AEs to specific organs and no apparent dose dependency or difference between placebo and active treatment was observed. Notably, no hypersensitivity or infusion reactions were noted and vital signs, body temperature and electrocardiograms were unchanged. Pharmacokinetics of PRS-080 were dose-proportional with a half-life of approximately 3 days. PRS-080 administration resulted in an immediate decrease in plasma hepcidin concentration, which was followed by an increase in serum iron concentration and transferrin saturation, or TSAT. As shown in the figure below, the duration of this response in iron and TSAT increased dose-dependently from about 25 hours at the lower dose to about 185 hours at the highest dose.
Phase 1b study in anemic stage 5 CKD patients
Based on this positive safety and pharmacological activity we initiated a phase 1 study in stage 5 CKD patients undergoing hemodialysis and suffering from FID-anemia in 2016. This study was governed and approved by the BfArM. It was a multi-center, randomized and double blinded phase 1b clinical trial studying single PRS-080 administrations at 3 dose levels, 2, 4, and 8 mg/kg bw. This study was completed in the first quarter of 2017. In each dose group 6 patients were treated with PRS-080 and 2 patients with placebo. PRS-080 was well tolerated and shown to be safe in all dose groups. There was only one SAE (worsening of dry gangrene in a patient with long standing diabetic foot syndrome). Vital signs, physical examination, and electrocardiograms were not affected by the treatment with PRS-080. No differences in the safety analysis were observed between placebo and drug. There was no evidence of immunogenicity in the SAD study.
Pharmacokinetics shows a dose-proportional increase for free and total PRS-080 with a dose peak plasma concentration at 1 hour, or Tmax, and a rapid decrease over the following 19 hours.
The magnitude of iron mobilization by PRS-080 was dose-dependent with increases in both serum iron concentration and TSAT following treatment. The maximal concentrations, or Cmax, of iron and TSAT were reached within 19 hours in all three dose groups. A return to baseline values were reached after 2, 3, and 5 days after the end of infusions of 2, 4, and 8 mg/kg PRS-080, respectively. The figure below shows mean serum concentration of iron during the study:
The figure below shows mean TSAT during the study:
Phase 2a clinical study
Subsequently, and due to the good safety profile and iron mobilization of PRS-080, a multi-center, placebo controlled ascending dose pilot phase 2a study was initiated to examine the safety, PK and effects of hepcidin inhibition on iron mobilization by 5 weekly doses of PRS-080 in 4 and 8 mg/kg patient cohorts. In addition, first results on hemoglobin levels in CKD patients were examined as a secondary outcome measure. In total, 12 patients were included; in each dosage level group 4 patients were treated with PRS-080 and 2 patients were treated with placebo. The study was initiated in September 2017 and the last patient was dosed in December 2018. We intend to present the full data set from this study in 2019. We also plan to share these data with ASKA, at which point ASKA will decide whether to exercise its option to develop and commercialize PRS-080 in Japan and other Asian territories. Additionally, we plan to share the dataset with others for potential partnerships outside of the ASKA territories.
Competition
The pharmaceutical and biotechnology industries are characterized by rapidly-advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our technology, development experience, scientific knowledge and strategies provide us with competitive advantages, we face and will continue to face intense competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions, both in the United States and worldwide.
We compete, or will compete, with existing and new therapies that may become available in the future. Some of these competitors are pursuing the development of pharmaceuticals that target the same diseases and conditions that our drug candidates target. Any drug candidates that we are able to develop and commercialize will compete with existing and new drugs being developed by our competitors. Our competitors may develop or market products or other novel technologies that are more effective, safer, more convenient or less costly than any that may be commercialized by us or may obtain regulatory approval for their products more rapidly than we may obtain approval for ours.
The acquisition or licensing of pharmaceutical products is also very competitive, and a number of more established companies, some of which have acknowledged strategies to license or acquire products and many of which are bigger than us and have more institutional experience and greater cash flows than we have, may have competitive advantages over us, as may other emerging companies taking similar or different approaches to product licenses and/or acquisitions. In addition, a number of established research-based pharmaceutical and biotechnology companies may acquire products in late stages of development to augment their internal product lines, which may provide those companies with an even greater competitive advantage.
There are a number of other companies presently working to develop therapies for respiratory diseases and cancer, including divisions of large pharmaceutical companies and biotechnology companies of various sizes. There are also a variety of available drug therapies marketed for these diseases. Our drug candidates, if any are approved, may compete with these existing drug and other therapies, and to the extent they are ultimately used in combination with or as an adjunct to these therapies, our drug candidates may not be competitive with them. Some of the currently approved drug therapies are branded and subject to patent protection, and others are available on a generic basis. Many of these approved drugs are well-established
therapies and are widely accepted by physicians, patients and third-party payors. As a result, market acceptance of, and a significant share of the market for, any of our drug candidates that we successfully introduce to the market will pose challenges.
In addition to currently marketed therapies, there are also a number of drugs in clinical development to treat respiratory diseases and cancer. These medicines in development may provide efficacy, safety, convenience and other benefits that are not provided by currently marketed therapies and may not be provided by any of our current or future product candidates. As a result, they may provide significant competition for any of our product candidates.
Many of our competitors will have substantially greater financial, technical and human resources than we have. Additional mergers and acquisitions in the pharmaceutical industry may result in even more resources being concentrated in some of our competitors. Competition may increase further as a result of advances made in the commercial applicability of technologies and greater availability of capital for investment in these fields. Our success will be based in part on our ability to build, obtain regulatory approval for and market acceptance of, and actively manage a portfolio of drugs that addresses unmet medical needs and creates value in patient therapy.
In addition, our competitors may have a variety of drugs in development or awaiting market approval that could reach the market and become established before we have a product to sell. Our competitors may also develop alternative therapies that could further limit the market for any drugs that we may develop. Many of our competitors are using technologies or methods different or similar to ours to identify and validate drug targets and to discover novel small molecule drugs. Many of our competitors and their collaborators have significantly greater experience than we do in the following:
| |
• | identifying and validating targets; |
| |
• | screening compounds against targets; |
| |
• | performing preclinical and clinical trials of potential pharmaceutical products; and |
| |
• | obtaining regulatory clearances. |
In addition, many of our competitors and their collaborators have substantially greater advantages in the following areas:
| |
• | research and development resources; |
| |
• | manufacturing capabilities; and |
Smaller companies also may prove to be significant competitors, particularly through proprietary research discoveries and collaborative arrangements with large pharmaceutical and established biotechnology companies. Many of our competitors have products that have been approved by the FDA, or its foreign counterparts, or are in advanced development. We face competition from other companies, academic institutions, governmental agencies and other public and private research organizations for collaborative arrangements with pharmaceutical and biotechnology companies, in recruiting and retaining highly-qualified scientific and management personnel and for licenses to additional technologies. Developments by others may render our product candidates or our technologies obsolete. Our failure to compete effectively could have a material adverse effect on our business.
PRS-060
Like PRS-060, new developments for the treatment of uncontrolled moderate to severe asthma patients mainly include drug candidates targeting the Th2 pathway by interfering with IL-4/IL-13, IL-5 actions, IL-33 or TSLP. Such agents include mepolizumab (GSK, IL-5), reslizumab (Teva, IL-5), benralizumab (AstraZeneca, IL-5Rα), tezepelumab (Amgen/AstraZeneca, TSLP), etokimab (AnaptysBio, IL-33) and REGN-3500/SAR-440340 (Regeneron/Sanofi, IL-33. These drugs are in later clinical development than PRS-060 (tezepelumab, etokimab and REGN-3500/SAR-440340), or have been approved (mepolizumab, reslizumab, benralizumab) for severe eosinophilic asthma. Dupilumab (Sanofi/Regeneron, IL-4R) has been approved for severe to moderate asthma; the antibody omalizumab, directed against IgE, is also approved and marketed for the treatment of uncontrolled, moderate to severe asthma patients. However, in contrast to PRS-060, these antibodies are given to
patients through injection and distribute systemically through the blood stream. CSJ117 (Novartis), an inhaled fAb fragment that targets TSLP, is currently in phase 1 clinical development. There are a number of other companies presently marketing or developing other therapies for asthmatic patients.
IO programs
The rationale behind the multispecific tumor-targeted co-stimulatory molecules is to activate the immune system in the tumor microenvironment. Other companies that also develop multispecific drug candidates designed to activate the immune system in a tumor dependent manner by targeting a co-stimulatory receptor, such as 4-1BB, include Roche, Molecular Partners, Alligator Biosciences, Aptevo Therapeutics and Genmab, among others. Additionally, there are multiple of drug candidates in preclinical or clinical trials targeting other co-stimulatory receptors, either in a tumor dependent or monospecific manner, including OX40, CD40, GITR, CD27 and ICOS.
The first checkpoint inhibitor, ipilimumab, targeting CTLA-4 was approved for the treatment of melanoma patients in 2011 and is being marketed by Bristol-Myers Squibb. Nivolumab from Bristol-Myers Squibb was approved for the treatment of melanoma in 2014 as the first PD-1 inhibitor. Pembrolizumab from Merck & Co was the second PD-1 inhibitor to be approved and the first one in the US. In addition to nivolumab and prembrolizumab there are multiple approved checkpoint inhibitors targeting the PD-1/PD-L1 pathway, for example, those from Roche, AstraZeneca, Pfizer, and Merck KGaA.
There are also drug candidates in preclinical or clinical testing for other checkpoint targets such as LAG3, TIM3 and B7-H3. Companies developing either dual-checkpoint inhibitors or combinations of two or more monospecific checkpoint inhibitors includes Xencor, F-Star, Bristol-Myers Squibb, Merus, GSK, Novartis, Merck & Co among others.
Additionally, a number of other companies, such as Amgen, Affimed, Macrogenics, F-Star, Molecular Partners, Xencor, Immunocore and Zymeworks, also pursue other multispecific approaches in oncology, which therapies are in clinical or preclinical development.
PRS-343
PRS-343 is bispecific Anticalin-antibody fusion protein targeting 4-1BB and HER2. PRS-343 has a bifunctional proposed mode of action. It is designed to both promote 4-1BB clustering by bridging 4-1BB-positive T cells with HER2-positive tumor cells, and to thereby provide a costimulatory signal to tumor antigen-specific T cells and inhibit HER2 signaling. Other drug candidates targeting the co-stimulatory receptor 4-1BB include urelumab, which is being developed by Bristol-Myers Squibb, and utomilumab, which is being developed by Pfizer, both of which are currently in clinical development (Trialtrove, December 6, 2018). In the HER2-positive space, several companies are active with approved, clinical and preclinical drugs candidates. The most prominent company is Roche, having three approved drugs on the market through its subsidiary Genentech. The first drug from Roche targeting HER2 is trastuzumab, which has been marketed for treatment of breast cancer patients since 1998 and for gastric cancer patients since 2010. The two other drugs are pertuzumab and ado-trastuzumab emtansine which both are marketed for breast cancer patients. In addition to PRS-343, there are also other HER2 targeting drug candidates in clinical development designed to induce an immune response by bridging HER2-positive tumor cell with immune cells, for example, GBR 1302, a bispecific antibody targeting HER2 and CD3, from Glenmark (Pharmaprojects, December 6, 2018).
One company has publicly disclosed a competitor HER2 and 4-1BB bispecific program to PRS-343. MacroGenics presented data on a HER2 and 4-1BB bispecific during their R&D day on December 13th, 2016. In addition to MacroGenics other companies have also disclosed 4-1BB-based bispecific drug candidates. Roche and Molecular Partners have both presented data on bispecific drug candidates targeting fibroblast activation protein, or FAP, and 4-1BB. Alligator Bioscience together with Aptevo Therapeutics have disclosed a 4-1BB-based bispecific drug candidate targeting 5T4. However, the 4-1BB-based bispecific drug candidates targeting FAP or 5T4 does not constitute direct competition to PRS-343 since they are targeting a different tumor target and will, thus, likely be developed in different indications or patient populations.
PRS-344
PRS-344 is bispecific Anticalin-antibody fusion protein targeting 4-1BB and PD-L1. PRS-344 is, similar to PRS-343, designed to promote 4-1BB clustering by bridging 4-1BB-positive T cells with, in the case of PRS-344, PD-L1-positive tumor cells, and to thereby provide a costimulatory signal to tumor antigen-specific T cells. Furthermore, the direct, PD-L1- targeting activity of PRS-344 may provide an additional therapeutic benefit by checkpoint blockade. Multiple companies have publicly disclosed competing 4-1BB and PD-L1 bispecific programs, including, for example, Genmab in collaboration with BioNTech, Incyte in
collaboration with Merus, Inhibrx in collaboration with Elpiscience, F-Star, MacroGenics, and Numab. Merus announced on January 7, 2019, that the FDA accepted their IND application for their bispecific 4-1BB/PD-L1 program MCLA-14.
PRS-080
There are very few other drug candidates in development that interfere with hepcidin function or expression. Nucleic acid-based approaches that were in preclinical development by IONIS/Xenon and Alnylam have been suspended for unknown reasons. Noxxon’s RNA aptamer NOX-H94 has completed phase 2 clinical studies in cancer and ESRD patients. While an increase of hemoglobin values was seen in cancer patients, no such effect could be confirmed in the ESRD population. We are not aware of any ongoing development for NOX-H94. PRS-080 is significantly more potent and has a longer half-life than NOX-H94. We therefore believe that Noxxon’s results are not predictive for efficacy of PRS-080. Lilly has been developing an antibody against hepcidin in cancer as well as CKD patients as well as an antibody against the FPN transporter. The latter has been suspended for unknown reasons and there has been no update on the anti-hepcidin antibody from Lilly since 2015. FerruMax develops a soluble form of hemojuvelin, a protein that regulates hepcidin expression and iron metabolism that aims to suppress the production rate of hepcidin.
There are also a number of companies which are focused on treating anemia in CKD patients under alternative approaches. Fibrogen (in partnership with Astellas and AstraZeneca), Akebia Therapeutics (in partnership with Mitsubishi Tanabe and Otsuka), GSK, Bayer, Daiichi Sankyo, Zydus Cadila and Japan Tobacco have hypoxia-inducible-factor prolyl hydroxylase, or HIF-PH, inhibitors in clinical development that target stimulation of bone marrow activity. Fibrogen reported positive results from phase 3 studies in China with its HIF-PH inhibitor roxadustat (December 2018) and it was approved in China for the treatment of patients with anemia caused by CKD in patients who are dialysis-dependent in December 2018. For the HIF-PH inhibitor field, Fibrogen’s roxadustat was approved in China at the end of 2018 (partnered with AstraZeneca), GSK reported positive phase 3 results in Japan for daprodustat and Akebia reported positive results from a phase 2 study in Asia with vadadustat (January 2018). Multiple phase 3 studies are currently ongoing with different HIF-PH inhibitors in both dialysis-dependent as well as dialysis-independent CKD patients. Launches in the US are currently expected for 2020 for several of such therapies. There are also various companies conducting late-stage development of erythropoietin biosimilars.
Manufacturing
We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We currently rely and expect to continue to rely on third-party contract manufacturers, or CMOs, for the manufacture of our drug candidates for larger scale preclinical and clinical testing, as well as for commercial quantities of any drug candidates that are approved.
We currently rely on multiple CMOs for all of our clinical supplies, including active pharmaceutical ingredients, or APIs, drug substances and finished drug products, and label and packaging for our preclinical research and clinical trials, including the phase 1 study for PRS-060, the phase 1 studies for PRS-343 and the planned phase 1 study for PRS-344.
We believe that we will be able to contract with other CMOs to obtain APIs if our existing sources of APIs were no longer available or sufficient, but there is no assurance that APIs would be available from other CMOs on acceptable terms, on the timeframe that our business would require, or at all. We do not have supply commitments or other arrangements in place with our existing CMOs. We also do not currently have arrangements in place for redundant supply of bulk drug substance.
We do not have any current contractual relationships for the manufacture of commercial supplies of any of our drug candidates if they are approved, and we intend to enter into agreements with a CMO and one or more back-up manufacturers for the commercial production of our product candidates as they near potential approval.
Any drug products to be used in clinical trials and any approved product that we may commercialize will need to be manufactured in facilities, and by processes, that comply with the FDA’s cGMP requirements and comparable requirements of the regulatory agencies of other jurisdictions in which we are seeking approval. We currently employ internal resources to manage our CMOs.
We believe that PRS-060, PRS 343 and PRS-344 and our other Anticalin-branded drug candidates can be manufactured in reliable and reproducible biologic processes from readily available starting materials. PRS-060 is produced using a bacterial expression system similar to those that have been used in the past for the production of other proteins and which systems are widely used in the industry. PRS-343 and PRS-344 are produced using mammalian expression systems similar to those systems that are widely used in the industry for the production of antibodies. We believe that the manufacturing process is amenable to scale-up and will not require unusual or expensive equipment. We expect to continue to develop, on our own or with our collaborators, drug candidates that can be produced cost-effectively at contract manufacturing facilities.
Intellectual Property and Exclusivity
Our commercial success depends in part on our ability to obtain and maintain exclusivity of our proprietary Anticalin technologies through intellectual property protection for our drug candidates, libraries of different protein scaffolds and consensus sequences, and the fundamental Anticalin platform technology, including novel therapeutic and diagnostic discoveries, as well as other proprietary know-how, and to operate without infringing on the intellectual property rights of others.
We seek to protect our exclusive position of Anticalin technologies by, among other means, prosecuting our own international, US, and foreign patent applications related to our proprietary technology, inventions, and improvements that are important to the development and implementation of our business. We have established intellectual property protection in relation to our Anticalin technologies in key global markets, including in North America, Europe and Asia. We also rely on trade secrets for confidential know-how, which we generally seek to protect through contractual (for example, confidentiality) agreements with employees and third parties.
We have protected the goodwill of our Company and our drug candidates, created through innovation and development, by putting in place trademark registrations of the Pieris and Anticalin marks as well as several defensive registrations.
We currently, and expect that we will continue to, file patent applications and maintain granted patents directed to our key drug candidates in an effort to establish intellectual property positions relating to new compositions of matter for these drug candidates, as well as novel medical applications of these compounds in the treatment, prevention or diagnosis of various indications. We also intend to seek patent protection, if available, with respect to biomarkers that may contribute to selecting the right patient population for the use of any of our drug candidates, or with respect to pharmaceutical formulations that may be useful to produce final medicinal products.
We own or are the exclusive licensee of a patent portfolio consisting of several issued US patents, and their respective counterparts in a number of foreign jurisdictions, including pending applications under the Patent Cooperation Treaty, pending US patent applications and corresponding pending patent applications in a number of foreign jurisdictions as well as pending provisional patent applications, as described in further detail below.
In applicable jurisdictions, we will seek patent term extensions for certain patents of ours, including the patent term adjustment period in the United States. If we obtain marketing approval for our drug candidates in the United States or certain jurisdictions outside of the United States, we may be eligible for regulatory protection, such as 12 years of data exclusivity for new biological entities in the United States and as mentioned below, up to five years of patent term extension potentially available in the United States, eight to 11 years of data and marketing exclusivity potentially available for new drugs in the European Union, up to five years of patent extension in Europe (supplemental protection certificate), and eight years of data exclusivity potentially available in Japan. There can be no assurance that we will qualify for any such regulatory exclusivity, or that any such exclusivity will prevent competitors from seeking approval solely on the basis of their own studies. See “Government Regulation.”
We hold issued patents and patent applications in the United States and other foreign jurisdictions, which patents are related to libraries of different scaffolds and consensus sequences such as human apolipoprotein D, human neutrophil gelatinase-associated lipocalin, or hNGAL, and human tear lipocalin, and are expected to expire between 2020 and 2030, subject to any patent term adjustments and terminal disclaimers in the United States. We also own a number of patents and patent applications at various stages of prosecution directed towards compositions of mater and in some cases, formulations or methods of use, of our preclinical and clinical drug candidates. Where possible, we will pursue patent term adjustments in the United States and any applicable foreign jurisdictions.
As a result of our research and licensing agreement, or the TUM License, with Technische Universität München, or TUM, we hold a worldwide exclusive license to multiple patents and patent applications. These patents and patent applications relate to Anticalin proteins specific for the T cell co-receptor CD4 and hNGAL lipocalin muteins and/or a library of an hNGAL scaffold of a certain consensus sequence, which patent is expected to expire in 2029, subject to any patent term adjustments or terminal disclaimers in the United States. We also hold an exclusive license to patents or patent applications related to bacterial lipocalin muteins and a1m lipocalin muteins.
We hold a number of pending patent applications and issued patents in the United States and foreign jurisdictions directed to newly-discovered or improved scaffold libraries of lipocalin muteins, compounds derived therefrom (i.e., specific drug candidates), or the uses of such compounds to treat, prevent and mitigate certain diseases and conditions whose pathological development involve the targets of interest as well as to diagnose, prognose and select treatments for the diseases and
conditions. We would expect that these patents and any patents that may issue from pending applications would likely expire between 2029 and 2039 without taking into account possible patent term adjustments or other extensions, however, any and all of these patent applications may not result in issued patents, and not all issued patents may be maintained in force for their entire term. We are actively pursuing intellectual property protection for our IO drug candidates in key global markets that, if granted, could expire as late as 2039 or later depending on the date of the filing of such patent applications.
In addition to patents, we hold trademarks in the United States for the Pieris and Anticalin marks. Similarly, we hold their respective counterparts, either as registered trademarks or as pending applications, in a number of foreign jurisdictions. We expect that we will continue to look for trademark protection for the goodwill associated with our Company and our drug candidates in the countries or regions where we will have investment, research and development, sales or other activities.
We also rely upon unpatented trade secrets and know-how and continuing technological innovation to develop and maintain our competitive advantage. We strive to protect our proprietary information, in part, by using confidentiality agreements and/or invention assignment agreements with our collaborators, scientific advisors, employees and consultants. The confidentiality agreements are designed to protect our proprietary information and, in the case of agreements requiring invention assignment, to grant us ownership of technologies that are developed through a relationship with a third party. We also actively manage our publication and patent applications in that we only disclose information necessary to stir scientific interest or demonstrate patentability without materially compromising the secrecy of our valuable trade secrets and know-how. While we consider trade secrets and know-how to be a critical component of our intellectual property, trade secrets and know-how can be difficult to protect. In particular, with respect to our technology platform, we anticipate that these trade secrets and know-how will, over the course of time, be disseminated within the industry through independent development, the publication of journal articles describing the methodology and the movement of personnel skilled in the technology from academic to industry positions and vice versa. As a result, those proprietary trade secrets and know-how may lose their value to us over a period of time, and we may lose any competitive advantage afforded by them, as they become public knowledge.
Strategic Partnerships and Other License Agreements
Since 2007, we have entered into several strategic partnerships and other license or option agreements to complement our drug discovery and development. Specifically, we have entered into strategic partnerships with Servier, AstraZeneca and Seattle Genetics, or collectively, the Strategic Partnerships, and other non-strategic license or option agreements with ASKA and other biopharmaceutical companies, or collectively, the License Agreements. Under the Strategic Partnerships and License Agreements, we have developed and conducted or will develop and conduct selection and screening of drug candidates, as well as in vitro potency and efficacy testing, using our Anticalin-brand drug discovery platform, our Anticalin libraries, and other proprietary methods to generate, identify, and characterize drug candidates against certain biological targets associated with several diseases. The Strategic Partnerships have provided us with approximately $120.3 million in cash from upfront and milestone payments to date. With respect to discontinued agreements, we have no ongoing performance obligations, and do not expect to receive any significant additional consideration pursuant to those agreements.
Under our ongoing Strategic Partnerships and License Agreements, our partners are obligated to use commercially reasonable efforts to develop and commercialize drug candidates identified in the course of the collaboration. We are entitled to receive from our partners’ research, development and regulatory milestone payments and, in some cases, including in the Servier, AstraZeneca and Seattle Genetics collaborations, royalties on net sales for products developed and commercialized under these collaborations. With respect to our Strategic Partnerships, we have commercial rights, including the option to co-develop or co-commercialize one or more therapeutic programs with the applicable partners. We plan to continue to actively seek out additional collaboration partners that fit within our corporate development strategy.
The Strategic Partnerships represent our cornerstone collaborations in our key therapeutic areas of respiratory diseases and IO and include co-development and co-commercialization options. Certain terms and conditions of these Strategic Partnerships are summarized below.
Our collaboration with AstraZeneca
On May 2, 2017, we entered into the AstraZeneca Collaboration Agreement and a non-exclusive Anticalin platform technology license agreement with AstraZeneca, or the AstraZeneca Platform License, collectively referred to as the AstraZeneca Agreements, which became effective on June 10, 2017, following expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976. Under the AstraZeneca Agreements the parties will advance several novel inhaled Anticalin proteins.
Under the AstraZeneca Agreements, we received an upfront, non-refundable payment of $45.0 million. In addition, we initiated a phase 1 study for the PRS-060, or the AstraZeneca Lead Product, or AZD1402, in December 2017 for which we received a $12.5 million milestone payment. We are also eligible to receive research, development, commercial, sales milestone payments, and royalty payments. The total potential milestones are categorized as follows: research, development, and commercial milestones - up to $1.1 billion; and sales milestones - up to $1.0 billion. We may receive tiered royalties on sales of potential products commercialized by AstraZeneca and for co-developed products, gross margin share of worldwide sales, depending on our level of committed investment.
The term of each of the AstraZeneca Agreements ends upon the expiration of all of AstraZeneca’s payment obligations under such AstraZeneca Agreement. The AstraZeneca Collaboration Agreement may be terminated by AstraZeneca in its entirety for convenience beginning 12 months after its effective date upon 90 days’ notice or, if we have obtained marketing approval for the marketing and sale of a product, upon 180 days’ notice. Each program may be terminated at AstraZeneca’s option; if any program is terminated by AstraZeneca, we will have full rights to such program. The AstraZeneca Collaboration Agreement may also be terminated by AstraZeneca or us for material breach upon 180 days’ notice of a material breach (or 30 days with respect to payment breach), provided that the applicable party has not cured such breach by the permitted cure period (including an additional 180 days if the breach is not susceptible to cure during the initial 180-day period) and dispute resolution procedures specified in the applicable AstraZeneca Agreement have been followed. The AstraZeneca Collaboration Agreement may also be terminated due to the other party’s insolvency and may in certain instances be terminated on a product-by-product and/or country-by-country basis. Each party may also terminate an AstraZeneca Agreement if the other party challenges the validity of patents related to certain intellectual property licensed under such AstraZeneca Agreement, subject to certain exceptions for infringement suits, acquisitions and newly-acquired licenses. The AstraZeneca Platform License will terminate upon termination of the AstraZeneca Collaboration Agreement, on a product-by-product and/or country-by-country basis.
Our collaboration with Servier
On January 4, 2017, we entered into the Servier Collaboration Agreement and a non-exclusive Anticalin platform license agreement with Servier, or the Servier Platform License, collectively referred to as the Servier Agreements. Pursuant to the terms of the Servier Agreements, we, along with Servier, will initially pursue five bispecific therapeutic programs. These programs, which have been defined, may combine antibodies from the Servier portfolio with one or more Anticalin proteins based on our proprietary platform to generate innovative IO bispecific drug candidates. The collaboration may be expanded by up to three additional therapeutic programs. We also have the option to co-develop and retain commercial rights in the United States for up to four programs, including any potential expansion, while Servier will be responsible for development and commercialization of the other programs worldwide.
Under the Servier Agreements, we received an upfront payment of €30.0 million (approximately $32.0 million) and have achieved two preclinical milestones related to PRS-344. We may also receive additional development-dependent and commercial milestone payments for each program. The total development, regulatory and sales-based milestone payments to us could exceed €1.7 billion during the life of the collaboration and are dependent on the final number of projects pursued and the number of co-development options exercised by us. We will share preclinical and clinical development costs for each co-developed program with Servier. In addition, we will be entitled to receive tiered royalties up to low double digits on the sales of commercialized products in the Servier territories.
The term of each of the Servier Agreements ends upon the expiration of all of Servier’s payment obligations under such Servier Agreement. The Servier Agreements may be terminated by either of us for material breach upon 90 days’ or 120 days’ notice of a material breach, with respect to the Servier Collaboration Agreement and the Servier Platform License, respectively, provided that the applicable party has not cured such breach by the applicable 90-day or 120-day permitted cure period, and dispute resolution procedures specified in the applicable Servier Agreement have been followed. The Servier Agreements may also be terminated due to the other party’s insolvency or for a safety issue and may in certain instances be terminated on a product-by-product and/or country-by-country basis. The Servier Platform License will terminate upon termination of the Servier Collaboration Agreement, on a product-by-product and/or country-by-country basis.
Our collaboration with Seattle Genetics
On February 8, 2018, we entered into the Seattle Genetics Collaboration Agreement and a non-exclusive Anticalin platform technology license agreement with Seattle Genetics, or the Seattle Genetics Platform License, collectively referred to as the Seattle Genetics Agreements, pursuant to which the parties will develop multiple targeted bispecific IO treatments for solid tumors and blood cancers.
Under the terms of the Seattle Genetics Agreements, Seattle Genetics paid us a $30 million upfront fee, will pay tiered royalties on net sales up to the low double-digits, and will pay us up to $1.2 billion in total success-based payments across three product candidates. The companies will pursue multiple antibody-Anticalin fusion proteins during a research phase, and Seattle Genetics has the option to select up to three therapeutic programs for further development. Prior to the initiation of a pivotal trial, we may opt into global co-development and US commercialization of the second program and share in global costs and profits on a 50/50 basis. Seattle Genetics will solely develop, fund and commercialize up to two other programs. Seattle Genetics may also decide to select additional candidates from the initial research phase for further development in return for the payment to us of additional fees, milestone payments, and royalties.
The term of each of the Seattle Genetics Agreements ends upon the expiration of all of Seattle Genetics’s payment obligations under such Seattle Genetics Agreement. The Seattle Genetics Collaboration Agreement may be terminated by Seattle Genetics on a product-by-product for convenience beginning 12 months after its effective date upon 90 days’ notice or, for any program where a pivotal study has been initiated, upon 180 days’ notice. Any program may be terminated at Seattle Genetics’s option. If any program is terminated by Seattle Genetics after a pre-defined pre-clinical stage, we will have full rights to continue such program. If any program is terminated by Seattle Genetics prior to such pre-defined pre-clinical stage, we will have the right to continue to develop such program but will be obligated to offer a co-development option to Seattle Genetics for such program. The Seattle Genetics Collaboration Agreement may also be terminated by Seattle Genetics or us for an uncured material breach by the other party upon 90 days’ notice, subject to extension for an additional 90 days if the material breach relates to diligence obligations and subject, in all cases, to dispute resolution procedures. The Seattle Genetics Collaboration Agreement may also be terminated due to the other party’s insolvency and may in certain instances, including for reasons of safety, be terminated on a product-by-product basis. Each party may also terminate the Seattle Genetics Agreements if the other party challenges the validity of any patents licensed under the Seattle Genetics Agreements, subject to certain exceptions. The Seattle Genetics Platform License will terminate upon termination of the Seattle Genetics Collaboration Agreement, whether in its entirety or on a product-by-product basis.
Our License Agreements are older than our Strategic Partnerships and relate to non-strategic therapeutic areas, or do not provide us with co-development and co-commercialization rights. A brief summary of certain terms of selected License Agreements are provided below.
Our ASKA Option Agreement
On February 27, 2017, we entered into the ASKA Option Agreement, granting ASKA an exclusive option to license development and commercial rights to our anemia drug, PRS-080, in Japan, South Korea and certain other Asian markets following completion of a multi-dose phase 2a study to be conducted by us in dialysis-dependent anemia patients.
Under the terms of the ASKA Option Agreement, we received an option payment of $2.75 million from ASKA. Following an analysis period after the completion of the ongoing phase 2a study conducted by us, ASKA may exercise its option to obtain an exclusive license to develop and commercialize PRS-080 in Japan, South Korea and certain other Asian markets (excluding China). Should ASKA exercise the option, we would be eligible for more than $80 million in combined option exercise fee and milestones associated with development and commercialization of PRS-080 in the first indication in Japan. We may receive further development milestones in additional indications, as well as in other countries within the ASKA territory. We may also receive double-digit royalties on net sales of PRS-080 up to the mid- to high-teens.
The term of the ASKA Option Agreement, including the option rights granted therein, ends on the earlier of (i) ASKA’s written notice to us of ASKA’s decision not to exercise the option rights granted under the ASKA Option Agreement, (ii) ASKA’s failure to exercise its option rights within 60 days after the final results of the phase 2a study are made available to ASKA, (iii) three months from date on which we deliver to ASKA the final results of the phase 2a study in the European Union, or (iv) our and ASKA’s execution of the definitive agreements granting ASKA licenses to develop and commercialize PRS-080 in Japan, South Korea and certain other Asian countries (excluding China) as contemplated under the ASKA Option Agreement.
Our collaboration with Roche
In December 2015, we entered into a research collaboration and license agreement, or the Roche Agreement, with F. Hoffmann- La Roche Ltd. and Hoffmann- La Roche Inc., or Roche, for the research, development, and commercialization of Anticalin-based drug candidates against a predefined, undisclosed target in cancer immune therapy selected by Roche. Roche notified us of the termination of the Roche Agreement, effective August 21, 2018. As a result, any Anticalin proteins generated prior to termination are wholly owned by us following termination of the Roche Agreement. Prior to the termination of the Roche Agreement, our platform technology successfully produced a number of discovery hits specific for the target from our
Anticalin libraries. Our drug supply agreement with Roche for access to atezolizumab, an approved PD-L1 inhibitor, for a combination study of PRS-343 and atezolizumab in HER2-positive cancer patients is not impacted by the termination of the Roche Agreement.
Our collaboration with Sanofi
In September 2010, we entered into a collaboration and license agreement with Sanofi, or the Sanofi Agreement, which was subsequently amended in February 2013. Under the terms of the Sanofi Agreement, we agreed to use our proprietary Anticalin technologies to identify drug candidates against certain targets, with further development and commercialization activities conducted by Sanofi. On June 18, 2018, Sanofi announced its divestment of its infectious disease unit to Evotec AG. Sanofi subsequently provided us notice of its termination of the Sanofi Agreement, effective August 23, 2018. In connection with the termination of the development of the P. aeruginosa program, and under the terms of the Sanofi Agreement, Sanofi will transfer all materials, data, and reports to us in connection with the return to us of the development program related to the P. aeruginosa program. We intend to diligently review the data associated with this program and consider our strategic options thereafter.
Our collaboration with Daiichi Sankyo
In May 2011, we entered into a collaboration research and technology licensing agreement, or the DS Agreement, with Daiichi Sankyo Company Limited, or Daiichi Sankyo or Daiichi, to research, develop and commercialize two Anticalin therapeutics. On May 8, 2017, Daiichi Sankyo discontinued the development of one of those two therapeutic programs, a PCSK9 program, or DS-9001, after having advanced this program to first-in-human studies for strategic reasons. Consequentially, this Anticalin program, including respective intellectual property rights, were transferred back to us. Due to Daiichi Sankyo’s strategic prioritization and commercial reasons related to competing calcitonin gene-related peptide, or CGRP, inhibitors in advanced stages of development, Daiichi Sankyo provided notice to us on March 1, 2018 of its termination of the second of the two therapeutic programs, a CGRP program. Pre-clinical data regarding this CGRP-antagonizing Anticalin protein indicate that it has good drug-like properties, including strong target affinity, the ability to neutralize the biological activity of CGRP in vitro and the ability to inhibit vasodilation, a cause of migraine pain, in rat skin following subcutaneous administration of the antagonist. In connection with the termination of the development of the CGRP program, and under the terms of the DS Agreement, Daiichi Sankyo was obligated to carry out certain activities to facilitate transfer of activities, regulatory filings, materials, data, agreements and other matters to us in connection with the return to us of the development program related to the CGRP program, and such transfer has been completed. We are diligently reviewing the data associated with the program and considering our strategic options.
In-License Agreements
In addition to the Strategic Licenses and Other License Agreements, we have in-licensed a number of technologies and therapeutics, hereinafter referred to as the In-License Agreements, to advance our pipeline and programs, some of which are described below.
TUM License
On July 4, 2003, we entered into our TUM License which was subsequently renewed and amended on July 26, 2007. The TUM License established a joint research effort led by Prof. Arne Skerra, Chair of Biological Chemistry of TUM, to optimize Anticalin technologies for use in therapeutic, prophylactic and diagnostic applications and as research reagents, and to gain fundamental insights in lipocalin scaffolds. We provided certain funding for TUM research efforts performed under the agreement. The research phase of this collaboration ended on February 28, 2013.
Under the terms of the TUM License, TUM assigned to us certain materials and records resulting from the research. We retained rights to inventions made by our employees, and TUM assigned to us all inventions made under the agreement jointly by our employees and TUM personnel, provided that our employees made certain inventive contributions. With respect to all other inventions made in the course of the research, TUM granted to us worldwide exclusive license rights under patents and patent applications claiming such inventions. TUM retained rights to practice these inventions for research and teaching purposes.
As a result of research efforts to date under the TUM License, we hold a worldwide exclusive license under our agreement with TUM to multiple patents and patent applications related to certain Anticalin proteins and libraries. We bear the costs of filing, prosecution and maintenance of patents assigned or licensed to us under the agreement.
As consideration for the assignments and licenses, we are obliged to pay to TUM license payments on development of our proprietary products claimed by patents assigned or licensed to us by TUM. For each of such proprietary products developed by us, we could be required to pay up to an aggregate of approximately €0.2 million ($0.2 million) in license payments to TUM under the agreement.
We also are obliged to pay low single-digit royalties, including annual minimum royalties, on sales of such products. Should we grant licenses or sublicenses to those patents to third parties, we are obliged to share a percentage of resulting revenue with TUM, which percentage of resulting revenue is creditable against our annual license payments to TUM. Our payment obligations are reduced by our proportionate contribution to a joint invention. Payment obligations terminate on expiration or annulment of the last patent covered by the agreement.
We can terminate the licenses to any or all licensed patents upon specified advance notice to TUM. TUM may terminate the license provisions of the agreement only for cause. Termination of the agreement does not terminate our rights in patents assigned to us.
Pieris and TUM initiated discussions in the second quarter of 2018 to clarify, expand and restructure the TUM License, including the parties’ obligations under such license agreement. The parties' recent discussions relate to revised commercial terms and to re-initiating additional collaborations between faculty at TUM and Pieris. While an amended and restated license agreement has not yet been completed, we intend to enter into such an amendment. We recorded the probable expected impact of the amendment in research and development expense in 2018, which is an increase in our financial obligations associated with the TUM License of approximately $2.3 million for amounts that would be due in 2019 for 2018 and 2017 sub-licensing activities. These discussions may also lead to an increase in our collaborative research activities with TUM.
Enumeral license agreements
In the second quarter of 2016, we entered into two license agreements, collectively the PD-1 In-License, with Enumeral Biomedical Holdings, Inc., or Enumeral, pursuant to which we in-licensed certain intellectual property related to an Enumeral-generated antibody against PD-1 and an option to in-license up to two additional antibodies against undisclosed targets. Under the PD-1 In-License, we acquired a non-exclusive worldwide license (except in the exclusive field of licensed antibodies fused to Anticalin proteins in the oncology area) under the applicable Enumeral patents and know-how owned by Enumeral to research, develop and commercialize fusion proteins incorporating Enumeral’s PD-1 antibody and one or more Anticalin proteins for use in the oncology area, or the Subsequent Options. The Subsequent Options expired on May 31, 2017. Enumeral also agreed not to practice or assist third parties in practicing in the exclusive field. On January 29, 2018, Enumeral filed a voluntary petition for relief under Chapter 11 of the United States Bankruptcy Code in the Bankruptcy Court for the District of Massachusetts, or the Bankruptcy Court. In connection with those proceedings, Enumeral transferred the intellectual property related to the PD-1 In-License to PD-1 Acquisition Group, LLC, or the Acquisition Group, who have assumed the rights and obligations of Enumeral with respect to the PD-1 In-License.
Under the terms of the PD-1 In-License, we are obliged to pay to Acquisition Group development and sales milestones on development of products incorporating the Enumeral antibody, as well as low to lower-middle single-digit royalties as a percentage of net sales depending on the amount of net sales in the applicable years. In the event that we are required to pay a license fee or royalty to any third party related to the licensed products, our royalty payment obligations to Acquisition Group will be reduced by the amount of such third-party fees or payments, up to 50% of the royalty payment for each calendar year due to Acquisition Group. Payment obligations terminate on a product-by-product and country-by-country basis on the later of 10 years from the first commercial sale of a product incorporating the Enumeral antibody or the last to expire, lapse or be abandoned of a claim from the licensed Enumeral patents filed as of the effective date of the PD-1 In-License that cover the manufacture, use, offer for sale, sale or import of a product incorporating the Enumeral antibody.
The term of the PD-1 In-License ends upon the expiration of the last to expire patent covered under the license unless earlier terminated by us or Acquisition Group in accordance with the terms of the PD-1 In-License.
Kelun license agreement
In connection with our efforts to develop multispecific Anticalin-based proteins designed to engage immunomodulatory targets, during the second quarter of 2017, we entered into a license and transfer agreement, or the Kelun Agreement, with Sichuan Kelun-Biotech Biopharmaceutical Co. Ltd., or Kelun. Under the Kelun Agreement, Kelun has granted to us a non-exclusive worldwide license (with the right to sublicense) under certain intellectual property owned or controlled by Kelun to research,
develop, manufacture, and commercialize bi- and multi- specific fusion proteins that include an antibody developed by Kelun specific for an undisclosed target and one or more Anticalin proteins.
Government Regulation
United States – FDA process
The research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing, among other things, of drug products are extensively regulated by governmental authorities in the United States and other countries.
US Drug development process
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or the FDCA, and in the case of biologics, also under the Public Health Service Act, or the PHSA, and implementing regulations. Failure to comply with the applicable US requirements may subject us to administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications, or NDAs, or biologics license applications, or BLAs, or their issuance of warning letters, or the imposition of fines, civil penalties, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution. The process required by the FDA before a drug or biologic may be marketed in the United States generally involves the following:
| |
• | completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices or other applicable regulations; |
| |
• | submission to the FDA of an IND which must become effective before human clinical trials may begin; |
| |
• | performance of adequate and well-controlled human clinical trials according to current good clinical practices, or cGCPs, to establish the safety and efficacy of the proposed drug for its intended use; |
| |
• | submission to the FDA of an NDA or BLA; |
| |
• | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMP to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| |
• | FDA review and approval of the NDA or BLA. |
Once a pharmaceutical candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND, which must become effective before human clinical trials may begin. An IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during studies due to safety concerns or non-compliance.
All clinical trials must be conducted under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing the objectives of the study, the parameters to be used in monitoring the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND. Further, an Institutional Review Board, or IRB, for each medical center proposing to conduct the clinical trial must review and approve the study protocol and informed consent information for study subjects for any clinical trial before it commences at that center, and the IRB must monitor the study until it is completed. Study subjects must sign an informed consent form before participating in a clinical trial. There are also requirements governing the reporting of on-going clinical trials and clinical trial results to public registries.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| |
• | Phase 1: The product candidate is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, such as cancer, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| |
• | Phase 2: This phase involves studies in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| |
• | Phase 3: Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product candidate and provide, if appropriate, an adequate basis for product labeling. |
The FDA may suspend clinical trials at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. The FDA may approve an NDA for a product candidate but require that the sponsor conduct additional clinical trials to further assess the drug after NDA approval under a post-approval commitment. Post-approval trials are typically referred to as phase 4 studies.
During the development of a new drug, sponsors are given opportunities to meet with the FDA at certain points. Including prior to submission of an IND, at the end of phase 2, and before submission of an NDA or BLA. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and the FDA to reach agreement on the next phase of development. Sponsors typically use the end of phase 2 meeting to discuss their phase 2 clinical results and present their plans for the pivotal phase 3 studies that they believe will support approval of the new drug.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
US review and approval processes
The results of product development, preclinical studies and other nonclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling, and other relevant information are submitted to the FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of user fees; a waiver of such fees may be obtained under certain limited circumstances. The FDA reviews all NDAs and BLAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an NDA or BLA for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA may refer the NDA or BLA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The approval process is lengthy and often difficult, and the FDA may refuse to approve an NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. The FDA may issue a complete response letter, which may require additional clinical or other data or impose other conditions that must be met to secure final approval of the NDA or BLA, or an approved letter following satisfactory completion of all aspects of the review process. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. The FDA reviews a BLA to determine, among other things whether the product is safe, pure and potent and the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product’s continued safety, purity and potency.
NDAs or BLAs receive either standard or priority review. A drug representing a significant improvement in treatment, prevention or diagnosis of disease may receive priority review. Priority review for an NDA for a new molecular entity and original BLAs will be six months from the date that the NDA or BLA is filed. In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform adequate and well-controlled post-marketing
clinical trials. Priority review and accelerated approval do not change the standards for approval but may expedite the approval process.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may require a sponsor to conduct phase 4 testing which involves clinical trials designed to further assess a drug’s safety and effectiveness after NDA or BLA approval and may require testing and surveillance programs to monitor the safety of approved products which have been commercialized.
The Food and Drug Administration Safety and Innovation Act, or the FDASIA, enacted in 2012, made permanent the Pediatric Research Equity Act, or the PREA, which requires a sponsor to conduct pediatric studies for most drugs and biologicals, for a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs, BLAs and supplements thereto, must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective.
Patent term restoration and marketing exclusivity
Depending upon the timing, duration and specifics of FDA approval of our drugs, some of our US patents may be eligible for limited patent term extension. These patent term extensions permit a patent restoration term of up to five years as compensation for any patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA or BLA, plus the time between the submission date of an NDA or BLA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension, and the extension must be applied for prior to expiration of the patent. The United States Patent and Trademark Office, or the USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
Pediatric exclusivity is another type of marketing exclusivity available in the United States. The FDASIA made permanent the Best Pharmaceuticals for Children Act, or the BPCA, which provides for an additional six months of marketing exclusivity if a sponsor conducts clinical trials in children in response to a written request from the FDA, or a Written Request. If a Written Request does not include studies in neonates, the FDA is required to include its rationale for not requesting those studies. The FDA may request studies on approved or unapproved indications in separate Written Requests. The issuance of a Written Request does not require the sponsor to undertake the described studies.
Biologics Price Competition and Innovation Act of 2009
In March 2010, the Patient Protection and Affordable Care Act was enacted in the United States and included the Biologics Price Competition and Innovation Act of 2009, or the BPCIA. The BPCIA amended the PHSA to create an abbreviated approval pathway for two types of “generic” biologics—biosimilars and interchangeable biologic products, and provides for a 12-year exclusivity period for the first approved biological product, or reference product, against which a biosimilar or interchangeable application is evaluated; however, if pediatric studies are performed and accepted by the FDA, the 12-year exclusivity period will be extended for an additional six months. A biosimilar product is defined as one that is highly similar to a reference product notwithstanding minor differences in clinically inactive components and for which there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity and potency of the product. An interchangeable product is a biosimilar product that may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.
The biosimilar applicant must demonstrate that the product is biosimilar based on data from (1) analytical studies showing that the biosimilar product is highly similar to the reference product; (2) animal studies (including toxicity); and (3) one or more clinical studies to demonstrate safety, purity and potency in one or more appropriate conditions of use for which the reference product is approved. In addition, the applicant must show that the biosimilar and reference products have the same mechanism of action for the conditions of use on the label, route of administration, dosage and strength, and the production facility must meet standards designed to assure product safety, purity and potency.
An application for a biosimilar product may not be submitted until four years after the date on which the reference product was first approved. The first approved interchangeable biologic product will be granted an exclusivity period of up to one year after it is first commercially marketed, but the exclusivity period may be shortened under certain circumstances.
Orphan drug designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years. Orphan drug exclusivity, however, also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug as defined by the FDA for treatment of the same indication or disease.
The FDA also administers a clinical research grants program, whereby researchers may compete for funding to conduct clinical trials to support the approval of drugs, biologics, medical devices, and medical foods for rare diseases and conditions. A product does not have to be designated as an orphan drug to be eligible for the grant program. An application for an orphan grant should propose one discrete clinical study to facilitate FDA approval of the product for a rare disease or condition. The study may address an unapproved new product or an unapproved new use for a product already on the market.
Fast-track designation and accelerated approval
The FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment, and which demonstrate the potential to address unmet medical needs for the condition. Under the fast-track program, the sponsor of a new drug candidate may request that the FDA designate the drug candidate for a specific indication as a fast-track drug concurrent with, or after, the filing of the IND for the drug candidate. The FDA must determine if the drug candidate qualifies for fast-track designation within 60 days of receipt of the sponsor’s request.
Under the fast-track program and the FDA’s accelerated approval regulations, the FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of phase 4 or post- approval clinical studies to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug from the market on an expedited basis. All promotional materials for drug candidates approved under accelerated regulations are subject to prior review by the FDA.
In addition to other benefits such as the ability to use surrogate endpoints and engage in more frequent interactions with the FDA, the FDA may initiate review of sections of a fast-track drug’s BLA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the fast-track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Post-approval requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws and regulations. We rely, and expect to continue to rely, on third parties to produce clinical and commercial quantities of our
products. Future inspections by the FDA and other regulatory agencies may identify compliance issues at the facilities of our CMOs that may disrupt production or distribution or require substantial resources to correct.
Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the drug, providing the FDA with updated safety and efficacy information, drug sampling and distribution requirements, complying with certain electronic records and signature requirements, and complying with FDA promotion and advertising requirements. The FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label.
From time to time, new legislation and regulations may be implemented that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. It is impossible to predict whether further legislative or regulatory changes will be enacted, or FDA regulations, guidance or interpretations changed or what the impact of such changes, if any, may be.
Foreign regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries or economic areas, such as the 28-member European Union, before we may commence clinical trials or market products in those countries or areas. It is not yet clear how the United Kingdom’s withdrawal from the European Union, effective March 2019, will affect the approval of medicinal products in the UK. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Under EU regulatory systems, a company may submit marketing authorization applications, or MAAs, either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for medicinal products produced by biotechnology or those medicinal products containing new active substances for specific indications such as the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, viral diseases and designated orphan medicines, and optional for other medicines which are highly innovative. Under the centralized procedure, a marketing application is submitted to the European Medicines Agency. or EMA, where it will be evaluated by the Committee for Medicinal Products for Human Use and a favorable opinion typically results in the grant by the European Commission of a single marketing authorization that is valid for all EU member states. The initial marketing authorization is valid for five years, but once renewed is usually valid for an unlimited period.
When conducting clinical trials in the EU, we must adhere to the provisions of the EU Clinical Trials Directive and the laws and regulations of the EU Member States implementing them. These provisions require, among other things, that the prior authorization of an ethics committee and the submission and approval of a clinical trial authorization application be obtained in each Member State be obtained before commencing a clinical trial in that Member State.
As in the United States, it may be possible in foreign countries to obtain a period of market and/or data exclusivity that would have the effect of postponing the entry into the marketplace of a competitor’s generic product. As in the United States, a sponsor may apply for designation of a product as an orphan drug for the treatment of a specific indication in the EU before the application for marketing authorization is made. Orphan drugs in Europe are afforded economic and marketing benefits, including up to 10 years of market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product.
Reimbursement
Sales of pharmaceutical products depend in significant part on the availability of third-party reimbursement. Third-party payors include government healthcare programs, managed care providers, private health insurers and other organizations. These third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. We may need to conduct expensive pharmacoeconomic studies to demonstrate the cost-effectiveness of our products. Our product candidates may not be considered cost-effective. It is time consuming and expensive to seek reimbursement from third-party payors. Reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides
options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to by significantly lower.
Description of the Acquisition
On December 17, 2014, Pieris Pharmaceuticals, Inc., Pieris GmbH and the former stockholders of Pieris GmbH entered into an acquisition agreement, or the Acquisition Agreement. Pursuant to the Acquisition Agreement, the stockholders of Pieris GmbH contributed all their equity interests in Pieris GmbH to Pieris in exchange for shares of Pieris common stock, which resulted in Pieris GmbH becoming a wholly-owned subsidiary of Pieris, which we refer to as the Acquisition.
Upon the closing of the Acquisition on December 17, 2014, Pieris ceased to be a “shell company” under applicable rules of the Securities and Exchange Commission, or the SEC.
Emerging growth company and smaller reporting company status
The Jumpstart Our Business Startups Act of 2012, or the JOBS Act, establishes a class of company called an “emerging growth company,” which generally is a company whose initial public offering was completed after December 8, 2011 and had total annual gross revenues of less than $1.07 billion during its most recently completed fiscal year. Additionally, Rule 12b-2 of the Exchange Act establishes a class of company called a “smaller reporting company,” which effective September 10, 2018, was amended to include companies with a public float of less than $250 million as of the last business day of its most recently completed second fiscal quarter or, if such public float is less than $700 million, had annual revenues of less than $100 million during the most recently completed fiscal year for which audited financial statements are available. For the year ended December 31, 2018, we qualify as both an emerging growth company and a smaller reporting company.
As an emerging growth company and a smaller reporting company, we are eligible and have taken advantage of certain extended accounting standards and exemptions from various reporting requirements that are not available to public reporting companies that do not qualify for those classifications, including, but not limited to, the following:
| |
• | Any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and financial statements, commonly known as an “auditor discussion and analysis.” |
| |
• | A requirement to hold non-binding advisory stockholder votes on executive compensation or any “golden parachute” payments not previously approved by stockholders. |
| |
• | A requirement to comply with the requirement of auditor attestation of internal controls over financial reporting, which is required for other public reporting companies by Section 404 of the Sarbanes-Oxley Act of 2002. |
| |
• | An opportunity for reduced disclosure obligations regarding executive compensation in its periodic and annual reports, including without limitation exemption from the requirement to provide a compensation discussion and analysis describing compensation practices and procedures. |
| |
• | An opportunity for reduced financial statement disclosure in its registration statements, which must include two years of audited financial statements rather than the three years of audited financial statements that are required for other public reporting companies. Smaller reporting companies are also eligible to provide such reduced financial statement disclosure in annual reports on Form 10-K. |
Emerging growth companies may also elect to take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
For as long as we continue to be an emerging growth company and/or a smaller reporting company, we expect that we will take advantage of the reduced disclosure obligations available to us as a result of those respective classifications. We will remain an emerging growth company until the earlier of (i) December 31, 2019, the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act; (ii) the last day of the fiscal year in which we have total annual gross revenues of $1.07 billion or more; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under applicable SEC rules. We will remain a smaller reporting company until we have a public float of $250 million or more as of the last business day of our most recently completed second fiscal quarter, and we could retain our smaller reporting company status indefinitely depending on the size of our public float.
Employees
As of December 31, 2018, we had 107 full-time employees and nine permanent part-time employees. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We consider our relationship with our employees to be good. In order to successfully develop our drug candidates, we must be able to attract and retain highly skilled personnel. We anticipate hiring additional employees for research and development, clinical and regulatory affairs and general and administrative activities over the next few years. We also utilize the services of consultants, clinical research organizations, and other third parties on a regular basis.
Available Information
Our Internet address is www.pieris.com. Copies of our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports, are available to you free of charge through the Investors section of our website as soon as reasonably practicable after such materials have been electronically filed with, or furnished to, the SEC. The information contained on, or that can be accessed through, our website is not part of this Annual Report on Form 10-K. We have included our website address in this Annual Report on Form 10-K solely as an inactive textual reference.
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, together with the other information contained in this Annual Report on Form 10-K, including our consolidated financial statements and the related notes, before making any decision to invest in shares of our common stock. This Annual Report on Form 10-K contains forward-looking statements. If any of the events discussed in the risk factors below occurs, our business, prospects, results of operations, financial condition and cash flows could be materially harmed. If that were to happen, the trading price of our common stock could decline, and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks not currently known to us or other factors not perceived by us to present significant risks to our business at this time also may impair our business operations.
Risks Related to our Business, Financial Position, Capital Requirements, Managing our Growth and other Legal Compliance Matters
We have incurred significant losses since our inception and anticipate that we will continue to incur losses for the foreseeable future. We currently have no product revenues and no approved products and will need to raise additional capital to operate our business.
We are a clinical-stage biopharmaceutical company. To date, we have not generated any commercial sales revenue and are not profitable and have incurred losses since our inception in August 2000. For the years ended December 31, 2018 and 2017 we reported net loss of $26.8 million and $17.6 million, respectively. As of December 31, 2018, we had an accumulated deficit of $147.1 million. We expect to continue to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our drug candidates and the commercialization of approved products, if any.
We are currently focused primarily on the development of, and our preclinical IO programs and drug candidates, our respiratory drug candidate PRS-060, currently in phase 1 studies in partnership with AstraZeneca, and our lead drug candidates and programs, our IO programs including PRS-343, currently in phase 1 studies, and our hepcidin antagonist, PRS-080, currently in phase 2a studies, as well as our other programs, including additional partnered programs with AstraZeneca, Servier and Seattle Genetics, which we believe will result in our continued incurrence of significant research, development and other expenses and resources related to those programs. If our research and development efforts, including preclinical studies or clinical trials for
any of our drug candidates fail or produce unsuccessful results and those drug candidates do not gain regulatory approval, or if any of our drug candidates, if approved, fail to achieve market acceptance, we may never become profitable. In addition, the failure of one drug candidate or program may have an adverse impact on other drug candidates and programs that include our class of Anticalin proteins. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ equity and working capital.
We are highly dependent on the success of PRS-060, our lead candidate in our respiratory pipeline, and PRS-343, the lead candidate in our IO pipeline. We are executing a broad development program for each of PRS-060 and PRS-343 and clinical and regulatory outcomes for each of PRS-060 and PRS-343, if not successful, will significantly harm our business.
Our future success is highly dependent on our ability to successfully develop, obtain regulatory approval for, and commercialize PRS-060 and PRS-343. In general, most early stage investigatory drugs, including inhaled therapeutics such as PRS-060 and oncology drug candidates such as PRS-343, do not become approved drugs. Accordingly, there is a very meaningful risk that PRS-060 and PRS-343 will not succeed in one or more clinical trials sufficient to support one or more regulatory approvals. To date, clinical and preclinical outcomes from PRS-060 and PRS-343 have had a significant impact on our market valuation, financial position, and business prospects, and we expect this to continue in future periods. If one or more clinical trials of PRS-060 or PRS-343 is not successful, it would materially harm our market valuation, prospects, financial condition and results of operations.
We will need substantial additional funding to continue our operations, which could result in significant dilution or restrictions on our business activities. We may not be able to raise capital when needed, if at all, or on terms acceptable to us, which would force us to delay, reduce or eliminate some or all of our product development programs or commercialization efforts and could cause our business to fail.
Our operations have consumed substantial amounts of cash since inception. We expect to need substantial additional funding to pursue the preclinical and clinical development of our drug candidates, as well as to launch and commercialize any drug candidates for which we receive regulatory approval.
We will require additional capital for the further development and commercialization of our drug candidates and programs and may need to raise additional funds sooner than we currently anticipate if we choose to and are able to expand more rapidly than we currently anticipate. Further, we expect our expenses to increase in connection with our ongoing activities, particularly as we advance additional programs through preclinical development and into the clinic and monitor their performance, and as we continue to advance and expand our preclinical and clinical programs, particularly PRS-060 as well as PRS-343 and PRS-080. In addition, if we obtain regulatory approval for any of our drug candidates, we expect to incur significant commercialization expenses related to regulatory requirements, product manufacturing, marketing, sales and distribution.
Furthermore, we expect to incur additional costs associated with operating as a public company. We may also encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may increase our capital needs and/or cause us to spend our cash resources faster than we expect.
To date, we have financed our operations through a mix of equity investments from private and public investors, the incurrence of debt, grant funding, and revenues from our various collaboration agreements, and we expect to continue to finance our operations through equity investments from public investors for the foreseeable future. Additional funding from those or other sources may not be available when or in the amounts needed, on acceptable terms, or at all.
If we raise capital through the sale of equity, or securities convertible into equity, it would result in dilution to our then existing stockholders, which could be significant depending on the price at which we may be able to sell our securities. If we raise additional capital through the incurrence of indebtedness, we would likely become subject to covenants restricting our business activities, and holders of debt instruments may have rights and privileges senior to those of our equity investors. In addition, servicing the interest and principal repayment obligations under debt facilities could divert funds that would otherwise be available to support research and development, clinical or commercialization activities.
If we obtain capital through collaborative arrangements, these arrangements could require us to relinquish rights to our Anticalin-brand technology or drug candidates and could result in our receipt of only a portion of the revenues associated with the partnered drug.
If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce, or eliminate our research and development for our drug candidates or any future commercialization efforts. Any of these events could significantly harm our business, financial condition, and prospects.
Our limited operating history as a clinical stage company may hinder our ability to successfully meet our objectives.
We were formed in August 2000, and since that time our focus has been on discovery of Anticalin-brand drug candidates. We are currently conducting clinical development of PRS-060 in partnership with AstraZeneca, PRS-343 and PRS-080, and are also advancing other drug candidates through preclinical development with the intention of initiating additional clinical-stage programs. In addition to our focus in IO and respiratory diseases, we are also exploring additional indications that may be suitable for Anticalin-brand drug therapeutics. Our drug candidates are in the early stages of development, have not obtained marketing approval, have never generated any revenue from sales, and will require extensive testing before commercialization. We have limited experience with clinical-stage operations, including manufacturing required to support clinical activities and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical area. In addition, the early-stage nature of our drug discovery and development operations can only provide limited operating results upon which investors can evaluate our business and prospects.
Our limited operating history may adversely affect our ability to implement our business strategy and achieve our business goals, which include, among others, the following activities:
| |
• | developing our drug candidates using unproven technologies; |
| |
• | undertaking preclinical development and clinical trials as well as formulating and manufacturing products; |
| |
• | obtaining the human, financial and other resources necessary to develop, test, manufacture, commercialize and market our drug candidates; |
| |
• | engaging corporate partners to assist in developing, testing, manufacturing and marketing our drug candidates; |
| |
• | continuing to build and maintain an intellectual property portfolio covering our technology and our drug candidates; |
| |
• | satisfying the requirements of clinical trial protocols, including patient enrollment, establishing and demonstrating the clinical safety and efficacy of our drug candidates and obtaining necessary regulatory approvals; |
| |
• | achieving acceptance and use by the medical community of our Anticalin platform and drug candidates after they receive regulatory approvals; |
| |
• | maintaining, growing and managing our internal teams as and to the extent we increase our operations and develop new segments of our business; |
| |
• | developing and maintaining successful collaboration, strategic and other relationships for the development and commercialization of our drug candidates that receive regulatory approvals with existing and new partners; and |
| |
• | managing our cash flows and any growth we may experience in an environment where costs and expenses relating to clinical trials, regulatory approvals and commercialization continue to increase. |
If we are unsuccessful in accomplishing these objectives, we may not be able to develop our drug candidates, raise capital, expand our business or continue our operations.
Our global operations subject us to various risks, and our failure to manage these risks could adversely affect our results of operations.
Our business is subject to certain risks associated with doing business globally. One of our growth strategies is to pursue opportunities for our business in several areas of the world, including the United States, Europe (including Germany) and Australia, any or all of which could be adversely affected by the risks set forth below. Accordingly, we face significant operational risks as a result of doing business internationally, such as:
| |
• | fluctuations in foreign currency exchange rates; |
| |
• | potentially adverse tax consequences and changes in tax laws; |
| |
• | challenges in providing solutions across a significant distance, in different languages and among different cultures; |
| |
• | different, complex and changing laws governing intellectual property rights, sometimes affording reduced protection of intellectual property rights in certain countries; |
| |
• | difficulties in staffing and managing foreign operations, particularly in new geographic locations, and related compliance with employment, immigration and labor laws for employees or other staff living abroad; |
| |
• | restrictions imposed by local labor practices and laws on our business and operations; |
| |
• | economic weakness, including inflation, or rapid changes in government, economic and political policies and conditions, political or civil unrest or instability, terrorism or epidemics and other similar outbreaks or events; |
| |
• | compliance with a wide variety of complex foreign laws, treaties and regulations; |
| |
• | unexpected changes in tariffs, trade barriers and other regulatory or contractual limitations on our ability to develop or sell our products in certain foreign markets; and |
| |
• | becoming subject to the laws, regulations and court systems of multiple jurisdictions. |
Our failure to manage the market and operational risks associated with our international operations could limit the future growth of our business and adversely affect our results of operations.
Our international operations pose currency risks, which may adversely affect our operating results and net income.
Our operating results may be affected by volatility in currency exchange rates and our ability to effectively manage our currency transaction risks. Our reporting currency is the US dollar, however, 66% of our operating expenses and all of our revenues come from operations outside of the United States. As such, our financial statements are translated for reporting purposes as follows: (1) asset and liability accounts at year-end rates, (2) income statement accounts at weighted average exchange rates for the year and (3) stockholders’ equity accounts at historical rates. Corresponding translation gains or losses are recorded in stockholders’ equity.
As we realize our strategy to expand in the United States, Germany, Australia and elsewhere internationally, our exposure to currency risks will increase. We do not manage our foreign currency exposure in a manner that would eliminate the effects of changes in foreign exchange rates. Therefore, changes in exchange rates between these foreign currencies and the US dollar will affect our revenues and expenses and could result in exchange losses in any given reporting period.
We incur currency transaction risks whenever we enter into either a purchase or a sale transaction using a currency other than the Euro, our functional currency, in particular our arrangements for the purchase of supplies or licensing and collaboration agreements with partners outside of the United States. In such cases, we may suffer an exchange loss because we do not currently engage in currency swaps or other currency hedging strategies to address this risk.
Given the volatility of exchange rates, we can give no assurance that we will be able to effectively manage our currency transaction risks or that any volatility in currency exchange rates will not have an adverse effect on our results of operations.
If we fail to comply with environmental, health and safety laws and regulations that apply to us, we could become subject to fines or penalties or incur costs that could harm our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of any hazardous materials we use and wastes we produce. The use of these materials in our business could result in contamination or injury, which could cause damage for which we may be responsible but may not have sufficient resources to pay. We also could incur significant costs associated with civil or criminal fines and penalties for failure to comply with these laws and regulations, which we may not be able to afford.
Although we maintain workers’ compensation insurance for our operations in Germany to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations applicable to us. These current or future laws and regulations may impair our research, development or production efforts or impact the research activities we pursue, particularly with respect to research involving human subjects or animal testing. Our failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions, which could cause our financial condition to suffer.
Health and safety regulations in the United States, Germany, and Australia and in the countries where our technology and potential products are developed, licensed or sold may prevent the sale or use of our technology or products in the future.
We are subject to a variety of regulations regarding worker health and safety in the United States, European Union (Germany), Australia, and in the countries where our technology and potential products are licensed or sold. Because our technology and potential products may frequently involve the manufacture or use of certain chemical or biological compounds, we are required to certify their safety for industrial use and development in a variety of countries and contexts. As there has not been sufficient testing to determine the long-term health and environmental risks of our Anticalin drug candidates and the materials used in the production of such drug candidates and any future products, future regulations may ban the use of our products due to the potential risk they pose to workers or may limit the use of our drug candidates in research and commercial settings. Any such regulations may have a substantial negative impact on our business and revenues and may cause our business to fail. Because we cannot guarantee the long-term safety of use or exposure to materials used during development or manufacture of our products, we may face liability for health risks or harms caused as a result of developing, manufacturing or other processes that use such materials. Any such claims may have a negative impact on our revenues and may prove substantially disruptive to our business in the future.
In addition, under the EU regulation on classification, labeling and packaging of substances and mixtures, or CLP, and under other regulations in the United States or other countries related to the clinical development of our drug candidates (including, for example, submissions to regulatory authorities such as the FDA and EMA as well as submissions related to obtaining a non-proprietary, or INN and USAN, name for our clinical drug candidates to the World Health Organization, or the WHO, and United States Adopted Name Council, or the USAN Council), we may be required to publicly disclose the composition of our proprietary products or substances, which may facilitate infringement or avoidance of our intellectual property by third parties and may potentially reduce the margin we are able to charge for our products by allowing competitors to more accurately determine our production costs. Future development of these regulations may have a further negative impact on our revenues and a substantial negative impact on our business.
We may be limited in our use of our net operating loss carryforwards.
As of December 31, 2018, we had net operating loss carryforwards for United States federal income tax purposes of $15.3 million and net operating loss carryforwards for state income tax purposes of $20.1 million. Tax loss carryforwards that were created prior to December 31, 2017 expire through 2037, all tax loss carryforwards created after that date do not expire. In the United States, utilization of the net operating loss carryforwards may be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, and similar state provisions due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of net operating loss carryforwards that can be utilized annually to offset future taxable income and tax, respectively. If we were to lose the benefits of these loss carryforwards, our future earnings and cash resources would be materially and adversely affected. We have not currently completed a study to assess whether an ownership change has occurred, or whether there have been multiple ownership changes since the Acquisition.
As of December 31, 2018, we had German corporate income tax and trade tax net operating loss carryforwards of approximately $80.3 million and $79.4 million, respectively, which may be used to reduce our future taxable income in Germany. Under current German laws, tax loss carryforwards may only be used to offset any relevant later assessment period (calendar year) by $1.2 million plus 60% of the exceeding taxable income and trade profit of such period. In addition, certain transactions, including transfers of shares or interest in the loss holding entity, may result in the partial or total forfeiture of tax losses existing at that date. Partial or total forfeiture of tax losses may further occur in corporate reorganizations of the loss holding entity.
Our business and operations would suffer in the event of system failures, and our operations are vulnerable to interruption by natural disasters, terrorist activity, power loss and other events beyond our control, the occurrence of which could materially harm our business.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, hacking, ransomware, cyber-attacks, unauthorized access as well as telecommunication and electrical failures. While we have not experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs and operations. For example, the loss of clinical trial data from completed, ongoing, or planned clinical trials could result in delays in our regulatory approval efforts and we may incur substantial costs to attempt to recover or reproduce such data. If any disruption or security breach resulted in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and/or the further development of our drug candidates could be delayed.
We are also vulnerable to accidents, electrical blackouts, labor strikes, terrorist activities, war and natural disasters and other events beyond our control, and we have not undertaken a systematic analysis of the potential consequences to our business as a result of any such events and do not have an applicable recovery plan in place. Except for our operations in Germany, where we have business interruption insurance against losses or damages resulting from fire, we do not carry other business interruption insurance that would compensate us for actual losses from interruptions of our business that may occur, and any losses or damages incurred by us could cause our business to materially suffer.
Our current operations are largely concentrated in two locations and any events affecting these locations may have material adverse consequences.
Our current operations are carried out primarily in our facilities located in Freising, Germany and Boston, Massachusetts. Any unplanned event, such as flood, fire, explosion, earthquake, extreme weather condition, medical epidemics, power shortage, telecommunication failure, or other natural or man-made accidents, or incidents that prevent us from fully utilizing our facilities in these two locations, may have a material adverse effect on our ability to operate our business, particularly on a daily basis, and have significant negative consequences on our financial and operating conditions. Loss of access to these facilities may result in increased costs, delays in the development of our product candidates, or interruption of our business operations. In the event of an accident or incident at these facilities, we cannot assure you that the amounts of our insurance will be sufficient to satisfy any damages and losses. If our facilities are unable to operate because of an accident or incident or for any other reason, even for a short period of time, any or all of our research and development programs may be harmed. Any business interruption may have a material adverse effect on our business, financial position, results of operations, and prospects
Our failure to comply with data protection laws and regulations could lead to government enforcement actions, private litigation and/or adverse publicity and could negatively affect our operating results and business.
We are subject to data protection laws and regulations that address privacy and data security. The legislative and regulatory landscape for data protection continues to evolve, and in recent years there has been an increasing focus on privacy and data security issues. In the United States, numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws and federal and state consumer protection laws govern the collection, use, disclosure and protection of health-related and other personal information. Failure to comply with data protection laws and regulations could result in government enforcement actions, which could include civil or criminal penalties, private litigation and/or adverse publicity and could negatively affect our operating results and business. In addition, in May 2016, the EU Parliament adopted the comprehensive General Data Privacy Regulation, or the GDPR to, among other things, impose more stringent data protection requirements for processors and controllers of personal data and provide for greater penalties and fines for noncompliance, including fines in amounts up to €20 million or 4% of total worldwide annual turnover, whichever is higher. The GDPR became fully effective in May 2018. In addition, in 2018, California adopted a new privacy law, scheduled to go into effect on January 1, 2020, that borrows heavily from the GDPR. Complying with the enhanced obligations imposed by the GDPR and other applicable international and US privacy laws and regulations may result in significant costs to our business and require us to amend certain of our business practices. Further, enforcement actions and investigations by regulatory authorities related to data security incidents and privacy violations continue to increase. The future enactment of more restrictive laws, rules or regulations and/or future enforcement actions or investigations could have a materially adverse impact on us through increased costs or restrictions on our businesses, and noncompliance could result in regulatory penalties and significant legal liability.
Significant disruptions of information technology systems or security breaches could adversely affect our business.
We are increasingly dependent upon information technology systems, infrastructure and data to operate our business. In the ordinary course of business, we collect, store and transmit large amounts of confidential information (including, among other things, trade secrets or other intellectual property, proprietary business information and personal information). It is critical that we do so in a secure manner to maintain the confidentiality and integrity of such confidential information. We also have outsourced elements of our operations to third parties, and as a result we manage a number of third-party vendors who may or could have access to our confidential information. The size and complexity of our information technology systems, and those of third-party vendors with whom we contract, and the large amounts of confidential information stored on those systems, make such systems vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees, third-party vendors, and/or business partners, or to cyber-attacks by malicious third parties. Cyber-attacks are increasing in their frequency, sophistication and intensity, and have become increasingly difficult to detect. Cyber-attacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information.
Significant disruptions of our information technology systems, or those of our third-party vendors, or security breaches could adversely affect our business operations and/or result in the loss, misappropriation and/or unauthorized access, use or disclosure of, or the prevention of access to, confidential information, including, among other things, trade secrets or other intellectual property, proprietary business information and personal information, and could result in financial, legal, business and reputational harm to us. For example, any such event that leads to unauthorized access, use or disclosure of personal information, including personal information regarding our patients or employees, could harm our reputation, require us to comply with federal and/or state breach notification laws and foreign law equivalents, and otherwise subject us to liability under laws and regulations that protect the privacy and security of personal information. Security breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm of the type described above. While we have implemented security measures to protect our information technology systems and infrastructure, there can be no assurance that such measures will prevent service interruptions or security breaches that could adversely affect our business.
Recent US tax legislation and future changes to applicable US or foreign tax laws and regulations may have a material adverse effect on our business, financial condition and results of operations.
We are subject to income and other taxes in the United States and foreign jurisdictions. Changes in laws and policy relating to taxes or trade may have an adverse effect on our business, financial condition and results of operations. For example, on December 22, 2017, President Trump signed into law the Tax Cuts and Jobs Act, or the TCJA, which significantly reforms the Code. The TCJA, among other things, includes changes to US federal tax rates, imposes significant additional limitations on the deductibility of interest and net operating loss carryforwards, allows for the expensing of capital expenditures, and puts into effect the migration from a “worldwide” system of taxation to a territorial system. Our net deferred tax assets and liabilities were revalued at the newly enacted US corporate rate.
We continue to examine the impact this tax reform legislation in the United States may have on our business on an ongoing basis. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the TCJA is uncertain and our business and financial condition could be adversely affected. The impact of this tax reform on holders of our securities is also uncertain and could be adverse. This Annual Report on Form 10-K does not discuss any such tax legislation or the manner in which it might affect us or purchasers of our securities. We urge our investors to consult with their legal and tax advisors with respect to such legislation and the potential tax consequences of investing in our securities.
Generally, future changes in applicable US or foreign tax laws and regulations, or their interpretation and application could have an adverse effect on our business, financial conditions and results of operations.
There could be an adverse change or increase in the laws and/or regulations governing our business.
We are subject to various laws and regulations in different jurisdictions, and the interpretation and enforcement of laws and regulations are subject to change. We are also subject to different tax regulations in each of the jurisdictions where we conduct our business or where our management is located. We expect the scope and extent of regulation in the jurisdictions in which we conduct our business, or where our management is located, as well as regulatory oversight and supervision, to generally continue to increase. There can be no assurance that future regulatory, judicial and legislative changes in any jurisdiction will not have a material adverse effect on us or hinder us in the operation of our business.
Inadequate funding for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent our product candidates from being developed or commercialized in a timely manner
or otherwise prevent those agencies from performing normal business functions on which the operation of our business rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, including beginning on December 22, 2018, the US government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly affect the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological advances. In addition, the competition in the anemia, asthma and cancer markets is intense. We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, fully integrated pharmaceutical and biotechnology companies, smaller companies that are collaborating with larger pharmaceutical companies, academic institutions, and other public and private research organizations.
There are several third-party drug candidates that could be competitive with drug candidates in our pipeline.
Drug candidates interfering with the function of type 2 helper T cells, or Th2, the biological pathway for PRS-060, and thus competing with PRS-060, include those that are being developed by Sanofi/Regeneron (dupilumab), GSK (mepolizumab), Teva (reslizumab) and Amgen/AstraZeneca (tezepelumab). Drugs targeting immunomodulatory targets and thus competing with our 300-series programs include those that are currently marketed by Bristol-Myers Squibb (ipilimumab, nivolumab), Merck & Co (pembrolizumab), Roche (atezolizumab), Merck Serono/Pfizer (avelumab) and AstraZeneca (durvalumab) and drug candidates being developed by Bristol-Myers Squibb (for example, urelumab/anti-CD137 and relatlimab/anti-LAG3), Pfizer (for example, utomilumab/anti-CD137 and PF-04518600/anti-OX40) and other clinical stage drug candidates also compete with our proprietary and partnered IO programs. Additionally, a number of other companies, such as Amgen, Affimed, Macrogenics, F-Star, Molecular Partners, Xencor, Immunocore and Zymeworks, also pursue multispecific approaches in oncology, which therapies are in clinical or preclinical development. Drug candidates interfering with hepcidin function and thus competing with PRS-080 include those that are being developed by Lilly (LY-2928057), FerruMax (FMX-8) and Ionis/Xenon (XEN701). In addition, drug candidates being develop for anemia associated with CKD targeting other pathways than hepcidin also compete with PRS-080, which includes HIF-PH inhibitors such as FibroGen/AstraZeneca/Astella’s roxadustat and Akebia/Mitsubishi/Otsuka’s vadadustat. For additional information about our third-party drug candidates that could be competitive with the drug candidates in our pipeline, see "Business--Competition."
These existing or future competing products may provide therapeutic convenience or clinical or other benefits for a specific indication greater than our products or may offer comparable performance at a lower cost. If our products fail to capture and maintain market share, we may not achieve sufficient product revenue and our business will suffer.
Many of our competitors have substantially greater financial, technical and other resources than we do, such as larger research and development staff and experienced marketing and manufacturing organizations, as well as significantly greater experience in:
| |
• | undertaking preclinical testing and clinical trials; |
| |
• | obtaining FDA and other regulatory approvals of drugs; |
| |
• | prosecuting and enforcing intellectual property rights; |
| |
• | formulating and manufacturing drugs; and |
| |
• | launching, marketing and selling drugs. |
Established pharmaceutical companies may invest heavily to accelerate discovery and development of or in-license novel compounds that could make our drug candidates less competitive. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA, EMA or other regulatory approval, or discovering, developing and commercializing medicines before we do, which would have a material adverse effect on our business and ability to achieve profitability from future sales of our approved drug candidates, if any. For additional information about our competitors, please see "Business--Competition."
We could be subject to product liability lawsuits based on the use of our drug candidates in clinical testing or, if obtained, following marketing approval and commercialization. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to cease clinical testing or limit commercialization of our drug candidates.
We could be subject to product liability lawsuits if any drug candidate we develop allegedly causes injury or is found to be otherwise unsuitable for human use during product testing, manufacturing, marketing or sale. Any such product liability claim may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against these claims, we will incur substantial liabilities or be required to limit commercialization of our product candidates. Even successful defense would require significant financial and management resources.
Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the clinical testing and commercialization of products we develop on our own or with collaborators. While we currently carry insurance that we believe is appropriate for a company at our stage of development, including with respect to our ongoing clinical trials and studies, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer.
In the future, we will seek to obtain appropriate insurance coverage with respect to any future clinical trials of our other drug candidates, but we may not be able to obtain the levels of coverage desired on acceptable terms, or at all. If we do secure product liability insurance, we may subsequently determine that additional amounts of coverage would be desirable at later stages of clinical development of our drug candidates or upon commencing commercialization of any drug candidate that obtains required approvals, but we may not be able to obtain such additional coverage amounts when needed on acceptable terms, or at all. Unless and until we obtain such insurance, we would be solely responsible for any product liability claims relating to our preclinical and clinical development activities. Further, even after any such insurance coverage is obtained, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by any insurance policies we may then have or that is in excess of the limits of our insurance coverage. We would be required to pay any amounts awarded by a court or negotiated in a settlement that exceed the coverage limitations or that are not covered by any product liability insurance we may obtain, and we may not have, or be able to obtain, sufficient capital to pay such amounts. A successful product liability claim or series of claims brought against us could cause our stock price to fall and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business and our prospects.
We will need to grow the size of our organization, and we may not successfully manage any growth we may achieve.
Our success will depend upon the expansion of our operations and our ability to successfully manage our growth. Our future growth, if any, may place a significant strain on our management and on our administrative, operational, and financial resources, requiring us to implement and improve our operational, financial, and management systems.
In addition, our ability to manage our growth effectively will hinge upon our ability to expand, train, manage, and motivate our employees. As of December 31, 2018, we have 107 full-time employees and nine permanent part-time employees. As our development and commercialization plans and strategies develop, these demands may also require the hiring of additional research, development, managerial, operational, sales, marketing, financial, accounting, legal, and other personnel.
Moreover, future growth could require the development of additional expertise by management and impose significant added responsibilities on members of management, including:
| |
• | effectively managing our clinical trials and submissions to regulatory authorities for marketing approvals; |
| |
• | effectively managing our internal research and development efforts such as discovery research and preclinical development; |
| |
• | identifying, recruiting, maintaining, motivating and integrating additional employees; |
| |
• | effectively managing our internal and external business development efforts with current or future partners, such as entering into additional collaboration arrangements and increasing out-licensing revenues; |
| |
• | establishing relationships with third parties essential to our business and ensuring compliance with our contractual obligations to such third parties; |
| |
• | developing and managing new divisions of our internal business, including any sales and marketing segment we elect to establish; |
| |
• | maintaining our compliance with public company reporting and other obligations, including establishing and maintaining effective internal control over financial reporting and disclosure controls and procedures; and |
| |
• | improving our managerial, development, operational and finance systems. |
We may not be able to accomplish any of those tasks, and our failure to do so could prevent us from effectively managing future growth, if any, and successfully growing our company.
Any increase in resources devoted to research and product development without a corresponding increase in our operational, financial, and management systems could have a material adverse effect on our business, financial condition and results of operations.
We may make future acquisitions that could disrupt our business, cause dilution to our stockholders and harm our financial condition and operating results.
We may, in the future, make acquisitions of, or investments in, companies that we believe have products or capabilities that are a strategic or commercial fit with our current business or otherwise offer opportunities for our company. In connection with these acquisitions or investments, we may:
| |
• | issue common stock or other forms of equity that would dilute our existing stockholders’ percentage of ownership; |
| |
• | incur debt and assume liabilities; and |
| |
• | incur amortization expenses related to intangible assets or incur large and immediate write-offs. |
We may not be able to complete acquisitions on favorable terms, if at all. If we do complete an acquisition, we cannot assure you that it will ultimately strengthen our competitive position or that it will be viewed positively by customers, financial markets or investors. Furthermore, future acquisitions could pose numerous additional risks to our operations, including:
| |
• | problems integrating the purchased business, products or technologies; |
| |
• | challenges in achieving strategic objectives, cost savings and other anticipated benefits; |
| |
• | increases to our expenses; |
| |
• | the assumption of significant liabilities that exceed the limitations of any applicable indemnification provisions or the financial resources of any indemnifying party; |
| |
• | inability to maintain relationships with key customers, vendors and other business partners of the acquired businesses; |
| |
• | diversion of management’s attention from their day-to-day responsibilities; |
| |
• | difficulty in maintaining controls, procedures and policies during the transition and integration; |
| |
• | entrance into marketplaces where we have no or limited prior experience and where competitors have stronger marketplace positions; |
| |
• | potential loss of key employees, particularly those of the acquired entity; and |
| |
• | that historical financial information may not be representative or indicative of our results as a combined company. |
The results of the United Kingdom’s referendum on withdrawal from the European Union may have a negative effect on global economic conditions, financial markets and our business.
In June 2016, the United Kingdom held a referendum in which voters approved an exit from the European Union, commonly referred to as “Brexit.” This referendum has created political and economic uncertainty, particularly in the United Kingdom and the European Union, and this uncertainty may persist for years. A withdrawal could, among other outcomes, disrupt the free movement of goods, services and people between the United Kingdom and the European Union, and result in increased legal and regulatory complexities, as well as potential higher costs of conducting business in Europe. The United Kingdom’s vote to exit the European Union could also result in similar referendums or votes in other European countries in which we do business. Given the lack of comparable precedent, it is unclear what financial, trade and legal implications the withdrawal of the United Kingdom from the European Union would have and how such withdrawal would affect us.
For example, Brexit could result in the United Kingdom or the European Union significantly altering its regulations affecting the clearance or approval of our product candidates that are developed in the United Kingdom or the European Union. Any new regulations could add time and expense to the conduct of our business, as well as the process by which our products receive regulatory approval in the United Kingdom, the European Union and elsewhere. In addition, the announcement of Brexit and the withdrawal of the United Kingdom from the European Union have had and may continue to have a material adverse effect on global economic conditions and the stability of global financial markets and may significantly reduce global market liquidity and restrict the ability of key market participants to operate in certain financial markets. Any of these effects of Brexit, among others, could adversely affect our business, our results of operations, liquidity and financial condition.
Given the lack of comparable precedent, it is unclear what financial, trade, regulatory and legal implications the withdrawal of the United Kingdom from the European Union would have and how such withdrawal would affect us. For example, we currently rely on multiple CMOs for all of our clinical supplies, including APIs, drug substances and finished drug products, and label and packaging for our preclinical research and clinical trials, including the phase 1 study for PRS-060, the phase 1 studies for PRS-343 and the phase 2a study for PRS-080, and any tariffs, differing regulatory requirements and other restrictions on the free movement of goods between the United Kingdom and the European Union that result from Brexit may have an adverse impact on this part of our supply chain. This could therefore negatively impact our clinical operations and, in particular, the advancement of our lead respiratory program, PRS-060, which would adversely affect our business, our results of operations and financial condition.
Risks Related to the Discovery and Development of our Drug Candidates
We are heavily dependent on the successful development of our drug candidates and programs and we cannot be certain that we will receive regulatory approvals or be able to successfully commercialize our products even if we receive regulatory approvals.
We currently have no products that are approved for commercial sale. We expect that a substantial portion of our efforts and expenditures over the next few years will be devoted to our respiratory programs including PRS-060, our other partnered programs with AstraZeneca, our proprietary respiratory programs, our proprietary IO programs, particularly PRS-343, our partnered programs with Servier, including PRS-344, our partnered programs with Seattle Genetics, PRS-080, our hepcidin antagonist, as well as our other programs. In partnership with AstraZeneca, PRS-060 is in clinical development with a phase 1 SAD study initiated in the last quarter of 2017 and a phase 1 MAD study initiated in July 2018. For PRS-343, a phase 1 study was initiated in the second quarter of 2017 and a phase 1 study of the drug candidate in combination with atezolizumab was initiated in the third quarter of 2018. We completed dosing of healthy volunteers in a phase 1a study with PRS-080 in June 2015, initiated a phase 1b study in the first quarter of 2016 (which was completed) and a phase 2a study in the last quarter of 2017. We are engaged in research and development activities with respect to a number of additional drug candidates and programs. All of our other drug candidates are in the discovery or early preclinical to IND-enabling stage. Accordingly, our business is currently substantially dependent on the successful development, clinical testing, regulatory approval and commercialization of PRS-060, PRS-343, PRS-080 and our other IO programs, which may never occur.
Before we can generate any revenues from sales of our lead drug candidates, we must complete the following activities for each of them, any one of which we may not be able to successfully complete:
| |
• | conduct additional preclinical and clinical development; |
| |
• | manage preclinical, manufacturing and clinical activities; |
| |
• | obtain regulatory approval; |
| |
• | establish manufacturing relationships for the clinical and post-approval supply of the applicable drug candidate; |
| |
• | build a commercial sales and marketing team, either internally or by contract with third parties; |
| |
• | develop and implement marketing strategies; and |
| |
• | invest significant additional cash in each of the above activities. |
Clinical testing of PRS-060 and PRS-343 was initiated in 2017 and clinical testing of PRS-080 is ongoing, while clinical testing for other programs including our IO programs, has not yet commenced, and the results of any future clinical trials or preclinical studies of these programs, if unsuccessful, could lead to our abandonment of the development of those drug candidates. If studies of these drug candidates produce unsuccessful results and we are forced or elect to cease their development, our business and prospects would be substantially harmed.
Preclinical and clinical testing of our drug candidates that have been conducted to date or will be conducted in the future may not have been or may not be performed in compliance with applicable regulatory requirements, which could lead to increased costs or material delays for their further development.
Given the complexity as well as the uncertainty inherent in biopharmaceutical preclinical and other nonclinical studies and clinical trials, and because of our limited operating experience, we may discover that our own development activities are not in compliance with applicable regulatory requirements or are otherwise deficient, and therefore, determine that the development of our drug candidates on the basis of those trials and studies is not warranted or will be delayed.
We have also entered into license, partnership, and option arrangements, such as with Servier, ASKA, AstraZeneca, and Seattle Genetics relating to certain of our drug candidates and may continue to do so in the future. Under some of these arrangements, the development of some of those drug candidates has been, or in the future may be, conducted wholly by such partners or third parties with which the partners contract. As a result, we have not been or may not be closely involved with or have any control over those development activities. Although some of such partners have provided information regarding those drug candidates and the related studies conducted to date, including certain data that is included in this Annual Report on Form 10-K, we have not received and may not receive in the future comprehensive information regarding all of those development activities, including the raw data from certain studies that have been conducted, information regarding the design, procedural implementation and structure and information regarding the manufacture of the drug candidates used in the studies. Because we may have limited or no input on the development of these drug candidates, we may discover that all or certain elements of the trials and studies our partners have performed have not been, or may not in the future be, in compliance with applicable regulatory standards or have otherwise been or may be deficient, and that advancement of the development of these drug candidates on the basis of those trials and studies is not warranted.
Further, the majority of our development activities for each of our drug candidates to date, including our phase 1 study with PRS-060, which is being conducted in Australia, our phase 1 study with PRS-343, which is currently being conducted in the United States, and our phase 1 and phase 2 studies with PRS-080, which are being conducted in Germany, and our anticipated future clinical trials, have been, are being, or may in the future be conducted in or outside the United States, including in Europe or Australia, and we may conduct some of our future development activities in other countries or regions. As a result, although those studies may meet the standards of applicable foreign regulatory bodies, the structure and design of those clinical trials and preclinical studies may not meet applicable FDA requirements and also may not meet the requirements of the applicable regulatory authorities in other foreign countries in which we desire to pursue marketing approval.
If the studies conducted by us or our partners or collaborators do not comply with applicable regulatory requirements or are otherwise not eligible for continued development in the United States, then we or our partners may be forced to conduct new studies in order to progress the development of our drug candidates. We, or our partners, may not have the funding or other resources to conduct or complete these additional studies, which would severely delay or prevent the development plans for these drug candidates and their commercialization. Any such deficiency and delay in the development of these drug candidates would significantly harm our business plans, product revenues and prospects.
Our research and development is focused on a rapidly evolving area of science, and our approach to drug discovery and development is novel and may never lead to marketable products.
Biopharmaceutical product development is generally a highly speculative undertaking and by its nature involves a substantial degree of risk. Our specific line of business, the discovery of Anticalin-brand drug therapeutics for patients with a variety of diseases and conditions, such as anemia, asthma and cancer, is an emerging field, and the scientific discoveries that form the
basis for our efforts to develop drug candidates are relatively new. Further, the scientific evidence to support the feasibility of developing drug candidates based on those discoveries is both preliminary and limited. In contrast to companies that focus on more traditional drug classes, such as antibodies and small molecules, we believe that we are the first, if not the only company, to work with Anticalin-brand drug therapeutics and work to advance these to a clinical stage of development. We are not aware of any company that has successfully developed and obtained approval for a drug based on Anticalin proteins. As a result, identifying drug targets based in part on their suitability with Anticalin-brand drug therapeutics, which is a fundamental aspect of our business approach, may not lead to the discovery or development of any drugs that successfully treat patients with the diseases and conditions we intend to target. Moreover, the lack of successful precedents in the development of Anticalin proteins could result in added complexities or delays in our development efforts. The failure of the scientific underpinnings of our business model to produce viable drug candidates would substantially harm our operations and prospects.
We may not be successful in our efforts to build a pipeline of drug candidates.
A key element of our strategy is to use and expand our Anticalin drug platform to build a pipeline of drug candidates to address different targets and advance those drug candidates through clinical development for the treatment of a variety of different types of diseases. Although our research efforts to date have resulted in identification of a series of targets, we may not be able to develop drug candidates that have good drug-like properties (target affinity, stability, half-life, etc.) and are safe and effective inhibitors or promoters of all or any of these targets. Even if we are successful in building a product pipeline, the potential drug candidates that we identify may not be suitable for clinical development for a number of reasons, including causing harmful side effects or demonstrating other characteristics that indicate a low likelihood of receiving marketing approval or achieving market acceptance. If our methods of identifying potential drug candidates fail to produce a pipeline of potentially viable drug candidates, then our success as a business will be dependent on the success of fewer potential drug candidates, which introduces risks to our business model and potential limitations to any success we may achieve.
Clinical drug development involves a lengthy and expensive process with uncertain outcomes, clinical trials are difficult to design and implement, and any of our clinical trials could produce unsuccessful results or fail at any stage in the process.
Clinical trials conducted on humans are expensive and can take many years to complete, and outcomes are inherently uncertain. Failure can occur at any time during the process. Additionally, any positive results of preclinical studies and early clinical trials of a drug candidate may not be predictive of the results of later-stage clinical trials, such that drug candidates may reach later stages of clinical trials and fail to show the desired safety and efficacy traits despite having shown indications of those traits in preclinical studies and early-stage clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier phases of the trials. Therefore, the results of any ongoing or future clinical trials we conduct may not be successful.
We initiated phase 1 studies for PRS-060 and PRS-343 in 2017 and initiated a phase 1 study of PRS-343 in combination with atezolizumab in 2018. In addition, we completed dosing of healthy volunteers in the clinical phase 1a study for PRS-080 in 2015, completed a phase 1b study in 2017 and initiated a phase 2a study in 2017. We may however experience delays in pursuing those or any other clinical trials, and any planned clinical trials may not begin on time, may require redesign, may not enroll sufficient healthy volunteers or patients in a timely manner, and may not be completed on schedule, if at all.
Clinical trials may be delayed for a variety of reasons, including delays related to:
| |
• | obtaining regulatory approval to commence a trial; |
| |
• | reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| |
• | obtaining IRB approval at each trial site; |
| |
• | enrolling suitable volunteers or patients to participate in a trial; |
| |
• | developing and validating companion diagnostics, if they are deemed necessary, on a timely basis; |
| |
• | changes in dosing or administration regimens; |
| |
• | failure of patients to complete a trial or return for post-treatment follow-up; |
| |
• | inability to monitor patients adequately during or after treatment; |
| |
• | clinical investigators deviating from trial protocols or dropping out of a trial; |
| |
• | regulators imposing a clinical hold due to observed safety findings or other reasons; and |
| |
• | inability to manufacture sufficient quantities of a drug candidate of acceptable quality for use in clinical trials. |
We rely and plan to continue to rely on CROs, CMOs and clinical trial sites to ensure the proper and timely conduct of our clinical trials. Although we have and expect that we will have agreements in place with CROs and CMOs governing their contracted activities and conduct, we will have limited influence over their actual performance. As a result, we ultimately do not and will not have control over a CRO or CMO’s compliance with the terms of any agreement it may have with us, its compliance with applicable regulatory requirements, or its adherence to agreed upon time schedules and deadlines, and a future CRO or CMO’s failure to perform those obligations could subject any of our clinical trials to delays or failure.
Further, we may also encounter delays if a clinical trial is suspended or terminated by us, by any IRB or ethics committee, by a Data Safety Monitoring Board, or DSMB, or by the FDA or EMA, or other regulatory authority. A suspension or termination may be due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements, inspection of the clinical trial operations or trial site by the FDA, EMA or other regulatory authorities, exposing participants to health risks caused by unforeseen safety issues or adverse side effects, development of previously unseen safety issues, failure to demonstrate a benefit from using a drug candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Therefore, we cannot predict with any certainty the schedule for commencement or completion of any currently ongoing, planned or future clinical trials.
If we experience delays in the commencement or completion of, or suspension or termination of, any clinical trial for our drug candidates, the commercial prospects of the drug candidate could be harmed, and our ability to generate product revenues from the drug candidate may be delayed or eliminated. In addition, any delays in completing our clinical trials will increase our costs, slow down our drug candidate development and approval process and jeopardize regulatory approval of our drug candidates and our ability to commence sales and generate revenues. The occurrence of any of these events could harm our business, financial condition, results of operations and prospects significantly.
If we experience delays or difficulties in the enrollment of research subjects in clinical trials, those clinical trials could take longer than expected to complete and our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for our drug candidates if we are unable to locate and enroll a sufficient number of research subjects to participate in these trials. In particular, for some diseases and conditions we are or will be focused on, our pool of suitable patients may be smaller and more selective and our ability to enroll a sufficient number of suitable patients may be limited or take longer than anticipated. In addition, some of our competitors have ongoing clinical trials for drug candidates that treat the same indications as our drug candidates, and volunteers or patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ drug candidates.
Patient enrollment for any of our clinical trials may also be affected by other factors, including without limitation:
| |
• | the severity of the disease under investigation; |
| |
• | the frequency of the molecular alteration we are seeking to target in the applicable trial; |
| |
• | the eligibility criteria for the clinical trial in question; |
| |
• | the perceived risks and benefits of the drug candidate under the clinical trial; |
| |
• | the extent of the efforts to facilitate timely enrollment in clinical trials; |
| |
• | the patient referral practices of physicians; |
| |
• | the ability to monitor volunteers or patients adequately during and after treatment; |
| |
• | the presence of other drug candidates in clinical development for the same indication or against the same target; and |
| |
• | the proximity and availability of clinical trial sites. |
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays and could require us to abandon one or more clinical trials altogether. Enrollment delays in our clinical trials may result in increased development costs for our drug candidates, and we may not have or be able to obtain sufficient cash to fund such increased costs when needed, which could result in the further delay or termination of the trial.
The review processes of regulatory authorities are lengthy, time consuming, expensive and inherently unpredictable. If we are unable to obtain approval for our drug candidates from applicable regulatory authorities, we will not be able to market and sell those drug candidates in those countries or regions and our business could be substantially harmed.
The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of drug products are, and will remain, subject to extensive regulation by the FDA in the United States and by the respective regulatory authorities in other countries where regulations differ. We are not permitted to market our drug candidates in the United States until we receive the respective approval of a BLA from the FDA, or in any foreign countries until we receive the requisite approval from the respective regulatory authorities in such countries. The time required to obtain approval, if any, by the FDA, EMA and comparable foreign authorities is unpredictable, but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. We have not submitted a marketing application such as a BLA to the FDA, an MAA to the EMA, or any similar application to any other jurisdiction. Moreover, we have not obtained regulatory approval for any drug candidate in any jurisdiction and it is possible that none of our existing drug candidates or any drug candidates we may seek to develop in the future will ever obtain regulatory approval.
Our drug candidates could fail to receive regulatory approval for many reasons, including any one or more of the following:
| |
• | the FDA, EMA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| |
• | we may be unable to demonstrate to the satisfaction of the FDA, EMA or comparable foreign regulatory authorities that a drug candidate is safe and effective for its proposed indication; |
| |
• | the results of clinical trials may not meet the level of statistical significance required by the FDA, EMA or comparable foreign regulatory authorities for approval; |
| |
• | we may be unable to demonstrate that a drug candidate’s clinical and other benefits outweigh its safety risks; |
| |
• | the FDA, EMA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| |
• | the data collected from clinical trials of our drug candidates may not be sufficient to support the submission of a BLA or other submission or to obtain regulatory approval in the United States or elsewhere; |
| |
• | the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies may fail to meet the requirements of the FDA, EMA or comparable foreign regulatory authorities; |
| |
• | the FDA, EMA or comparable foreign regulatory authorities may fail to approve the companion diagnostics we contemplate developing internally or with partners; and |
| |
• | the change of the standard of care or approval policies or regulations of the FDA, EMA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
The time and expense of the approval process, as well as the unpredictability of future clinical trial results and other contributing factors, may result in our failure to obtain regulatory approval to market, in one or more jurisdictions, PRS-060, PRS-343, PRS-080, our other respiratory and IO programs, our discovery stage programs, or any other drug candidates we are developing or may seek to develop in the future, which would significantly harm our business, results of operations and prospects. In such case, we may also not have the resources to conduct new clinical trials and/or we may determine that further clinical development of any such drug candidate is not justified and may discontinue any such programs.
In order to market and sell our products in any jurisdiction, we or our third-party collaborators must obtain separate marketing approvals in that jurisdiction and comply with its regulatory requirements. The review and approval procedures can vary drastically among jurisdictions, and each jurisdiction may impose different testing and other requirements to obtain and maintain marketing approval. Further, the time required to obtain those approvals, if any, may differ substantially among jurisdictions. In addition, in many countries or regions outside the United States, it is required that the product be approved for reimbursement before the product can be approved for sale in that country or region. Moreover, approval by the FDA or an equivalent foreign authority does not ensure approval by regulatory authorities in any other countries or regions. As a result, the ability to market and sell a drug candidate in more than one jurisdiction can involve significant additional time, expense and effort, and would subject us and our collaborators to the numerous and varying post-approval requirements of each jurisdiction governing commercial sales, manufacturing, pricing and distribution of our drug candidates. We or any third parties with whom we may collaborate may not have the resources to pursue those approvals, and we or they may not be able to obtain any approvals that are pursued. The failure to obtain marketing approval for our drug candidates in foreign jurisdictions could severely limit their potential market and ability to generate revenue.
On June 23, 2016, the electorate in the United Kingdom voted in favor of leaving the European Union, commonly referred to as Brexit. On March 29, 2017, the country formally notified the European Union of its intention to withdraw pursuant to Article 50 of the Lisbon Treaty. Since a significant proportion of the regulatory framework in the United Kingdom is derived from European Union directives and regulations, the referendum could materially impact the regulatory regime with respect to the approval of our product candidates in the United Kingdom or the European Union. Any delay in obtaining, or an inability to obtain, any marketing approvals in the United Kingdom, as a result of Brexit or otherwise, would prevent us from commercializing our product candidates in the United Kingdom and reduce our ability to generate revenue and achieve and sustain profitability. If any of these outcomes occur, we may be forced to restrict or delay efforts to seek regulatory approval in the United Kingdom for our product candidates, which could significantly and materially harm our business.
Furthermore, other European countries may seek to conduct referenda with respect to continuing membership with the European Union. We do not know to what extent Brexit or other comparable initiatives, or any resulting changes, would affect our ability to conduct clinical trials or obtain marketing approval in these jurisdictions, and each could materially impact our ability to conduct clinical trials or obtain marketing approval on a timely basis, or at all.
In addition, even if we were to obtain regulatory approval in one or more jurisdictions, regulatory authorities may approve any of our drug candidates for fewer or more limited indications than we request, may not approve prices we may propose to charge for our products, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a drug candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that drug candidate. Any of the foregoing circumstances could materially harm the commercial prospects for our drug candidates.
We may expend our limited resources to pursue a particular drug candidate or indication that does not produce any commercially viable products and may fail to capitalize on drug candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we must focus our efforts on particular research programs and drug candidates for specific indications. As a result, we may forgo or delay pursuit of opportunities with other drug candidates or for other indications that later prove to have greater commercial potential. Further, our resource allocation decisions may result in our use of funds for research and development programs and drug candidates for specific indications that may not yield any commercially viable products.
If we do not accurately evaluate the commercial potential or target market for a particular drug candidate, or if market conditions change, we may relinquish valuable rights to that drug candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such drug candidate. Any such failure to properly assess potential drug candidates could result in missed opportunities and/or our focus on drug candidates with low market potential, which would harm our business and financial condition.
Risks Related to our Dependence on Third Parties
We rely on third parties to conduct our clinical trials and preclinical studies. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or otherwise conduct the trials as required or comply with regulatory requirements, we may not be able to obtain regulatory approval for our drug candidates, commercialize our product candidates when expected or at all, and our business could be substantially harmed.
We depend upon independent investigators and contractors, such as CROs, universities and medical institutions, to conduct our clinical trials and preclinical studies. We rely upon, and plan to continue to rely upon, such third-party entities to execute our clinical trials and preclinical studies and to monitor and manage data produced by and relating to those studies and trials. However, in the future we may not be able to establish arrangements with CROs when needed or on terms that are acceptable to us, or at all, which could negatively affect our development efforts with respect to our drug candidates and materially harm our business, operations and prospects. As a result of the use of third-party contractors, we will have only limited control over certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies, including each of our clinical trials, is conducted in accordance with the applicable protocol, legal and regulatory requirements as well as scientific standards, and our reliance on any third-party entity will not relieve us of our regulatory responsibilities.
Based on our present expectations, we and our third-party contractors will be required to comply with cGCP for all of our drug candidates in clinical development. Regulatory authorities enforce cGCP through periodic inspections of trial sponsors, clinical investigators and trial sites. If we or any of our contractors fail to comply with applicable cGCP, the clinical data generated in the applicable trial may be deemed unreliable and the FDA, EMA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving a drug candidate for marketing, which we may not have sufficient cash or other resources to support and which would delay our ability to generate revenue from any sales of such drug candidate. Any agreements governing our relationships with CROs or other contractors with whom we currently engage or may engage in the future may provide those outside contractors with certain rights to terminate a clinical trial under specified circumstances. If such an outside contractor terminates its relationship with us during the performance of a clinical trial, we would be forced to seek an engagement with a substitute contractor, which we may not be able to do on a timely basis or on commercially reasonable terms, if at all, and the applicable clinical trial would experience delays or may not be completed.
If our contractors do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the data they obtain is compromised due to a failure to adhere to our clinical protocols, legal and regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for, or successfully commercialize, the affected drug candidates. In addition, we will be unable to control whether or not they devote sufficient time and resources to our preclinical and clinical programs. These outside contractors may not assign as great a priority to our programs or pursue them as diligently as we would if we were undertaking such programs ourselves. As a result, our operations and the commercial prospects for the effected drug candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed. These contractors may also have relationships with other commercial entities, some of whom may compete with us. If our contractors assist our competitors to our detriment, our competitive position would be harmed.
If our relationships with any third parties conducting our studies are terminated, we may be unable to enter into arrangements with alternative third parties on commercially reasonable terms, or at all. Switching or adding third parties to conduct our studies involves substantial cost and requires extensive management time and focus. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines. Although we carefully manage our relationships with third parties conducting our studies, we cannot assure you that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material and adverse effect on our business, financial condition and results of operations.
We rely and expect to continue to rely completely on third parties to formulate and manufacture our preclinical, clinical trial and commercial drug supplies. The development and commercialization of any of our drug candidates could be stopped, delayed or made less profitable if those third parties fail to provide us with sufficient quantities of such drug supplies or fail to do so at acceptable quality levels, including in accordance with applicable regulatory requirements or contractual obligations, and our operations could be harmed as a result.
We have no experience in drug formulation or manufacturing. We do not currently have, nor do we plan to acquire, the infrastructure or capability internally, such as our own manufacturing facilities, to manufacture our preclinical and clinical drug supplies for use in the conduct of our clinical trials and preclinical studies or commercial quantities of any drug candidates that may obtain regulatory approval. Therefore, we lack the resources and expertise to formulate or manufacture our own drug candidates. We have entered into agreements with CMOs for the clinical-stage manufacturing of certain of our drug candidates, including PRS-343, PRS-080 and others. We plan to enter into agreements with one or more manufacturers to manufacture, supply, store, and distribute drug supplies for our current and future clinical trials and/or commercial sales. We intend to establish or continue those relationships for the supply of our drug candidates; however, there can be no assurance that we will be able to retain those relationships on commercially reasonable terms, if at all. If we are unable to maintain those relationships, we could experience delays in our development efforts as we locate and qualify new CMOs. If any of our current drug candidates or any drug candidates we may develop or acquire in the future receive regulatory approval, we will rely on one or more CMOs to manufacture the commercial supply of such drugs.
Our reliance on a limited number of CMOs exposes us to the following risks:
| |
• | We may be unable to identify manufacturers on acceptable terms, or at all, because the number of qualified potential manufacturers is limited. Following BLA approval, a change in the manufacturing site could require additional approval from the FDA. This approval would require new testing and compliance inspections. |
| |
• | Our third-party manufacturers might be unable to formulate and manufacture our drugs in the volume and of the quality required to meet our clinical and commercial needs, if any. |
| |
• | Our future CMOs may not perform as contractually agreed or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store and distribute our products. |
| |
• | Drug manufacturers are subject to ongoing periodic unannounced inspection by the FDA, and some state agencies to ensure strict compliance with cGMP regulations and other US and corresponding foreign requirements. We do not have control over third-party manufacturers’ compliance with these regulations and standards. |
| |
• | If any third-party manufacturer makes improvements in the manufacturing process for our products, we may not own, or may have to share, the intellectual property rights to the innovation. |
Each of these risks could delay our clinical trials, the approval, if any, of our drug candidates or the commercialization of our drug candidates or result in higher costs or deprive us of potential product revenues.
Although our agreements with our CMOs require them to perform according to certain cGMP requirements such as those relating to quality control, quality assurance and qualified personnel, we cannot control the ability of our CMOs to implement and maintain these standards. If any of our CMOs cannot successfully manufacture material that conforms to our specifications and the regulatory requirements of the FDA, EMA or other comparable foreign authorities, we would be prevented from obtaining regulatory approval for our drug candidates unless and until we engage a substitute CMO that can comply with such requirements, which we may not be able to do. Any such failure by any of our CMOs would significantly impact our ability to develop, obtain regulatory approval for or market our drug candidates, if approved.
Further, we plan to rely on our manufacturers to purchase from third-party suppliers the materials necessary to produce our drug candidates for our clinical trials. We do not have, nor do we expect to enter into, any agreements for the commercial production of these raw materials, and we do not expect to have any control over the process or timing of our CMOs’ acquisition of raw materials needed to produce our drug candidates. Any significant delay in the supply of a drug candidate or the raw material components thereof for an ongoing clinical trial due to a manufacturer’s need to replace a third-party supplier of raw materials could considerably delay completion of our clinical trials, product testing and potential regulatory approval of our drug candidates. Additionally, if our future manufacturers or we are unable to purchase these raw materials to commercially produce any of our drug candidates that gains regulatory approvals, the commercial launch of our drug candidates would be delayed or there would be a shortage in supply, which would impair our ability to generate revenues from the sale of our drug candidates.
Disagreements with respect to the commercial terms of our sales, licensing, purchase or manufacturing agreements may limit our commercial success.
The rights and obligations of the partners to which we may license our Anticalin technology are governed by the licensing and collaboration agreements we enter into with those partners. In addition, our relationships with CROs and CMOs are governed by the service agreements between us and each manufacturer. Although we attempt to address the full range of possible events that may occur during the development or the manufacturing of Anticalin drug candidates and products, unanticipated or extraordinary events may occur beyond those contemplated by such agreements. Furthermore, our business relationships with our product manufacturers and our collaborators may include assumptions, understandings or agreements that are not included in our agreements with them, or that are inaccurately or incompletely represented by their terms. In addition, key terms in such agreements may be misunderstood or contested, even when both we and the other party previously believed that we had a mutual understanding of such terms.
Any differences in interpretation or misunderstandings between us and other parties may result in substantial costs and delays with respect to the development, manufacturing or sale of Anticalin drugs, and may negatively impact our revenues and operating results. Product manufacturers may fail to produce the products and partners may fail to develop the drug candidates with the diligence or under the timeline or in the manner we anticipated, and results may differ from the terms upon which we had agreed. As a result, we may be unable to supply drugs of the quality or in the quantity demanded or required. We may
suffer harm to our reputation in the market from missed development goals or deadlines and may be unable to capitalize upon market opportunities as a result. Resolution of these problems may entail costly and lengthy litigation or dispute resolution procedures. In addition, there is no guarantee that we will prevail in any such dispute or, if we do prevail, that any remedy we receive, whether legal or otherwise, will adequately redress the harm we have suffered. The delays and costs associated with such disputes may themselves harm our business and reputation and limit our ability to successfully compete in the market going forward.
We depend on third parties and intend to continue to license or collaborate with third parties, and events involving these strategic partners or any future collaboration could delay or prevent us from developing or commercializing products.
Our business strategy and our short- and long-term operating results depend in part on our ability to execute on existing strategic collaborations and to license or partner with new strategic partners. We have entered into and expect in the future to enter into collaborative arrangements with established pharmaceutical companies, which will lead, finance or otherwise collaborate with us or assist us in the development, manufacturing and marketing of our drug products. We believe collaborations allow us to leverage our resources and technologies and we anticipate deriving some revenues from research and development fees, license fees, milestone payments, and royalties from our collaborative partners.
Our prospects, therefore, may depend to some extent upon our ability to attract and retain collaborative partners and to develop technologies and products that meet the requirements of current or prospective collaborative partners. We have limited control over the amount and timing of resources that our current collaborators or any future collaborators devote to our collaborations or potential products, in particular with respect to our collaborations with AstraZeneca for the development of PRS-060 and with Servier for the development of PRS-344. These collaborators may breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities successfully and in a timely manner. Further, our collaborators may not develop or commercialize products that arise out of our collaborative arrangements or devote sufficient resources to the development, manufacturing, marketing or sale of these products. In addition, our collaborative partners may have the right to abandon research projects, guide strategy regarding prosecution of relevant patent applications and terminate applicable agreements, including funding obligations, prior to or upon the expiration of the agreed-upon research terms. By entering into such collaborations, we may preclude opportunities to collaborate with other third parties who do not wish to associate with our existing third-party strategic partners. In the event of termination of a collaboration agreement, termination negotiations may result in less favorable terms.
There can be no assurance that we will be successful in establishing collaborative arrangements on acceptable terms or at all, that collaborative partners will not terminate funding before the completion of projects, that our collaborative arrangements will result in successful product commercialization, or that we will derive any revenues from such arrangements. Potential collaborators may reject collaborations based upon their assessment of our financial, regulatory or intellectual property position and our internal capabilities. Additionally, the negotiation, documentation and implementation of collaborative arrangements are complex and time-consuming. Our discussions with potential collaborators may not lead to the establishment of new collaborations on favorable terms and may have the potential to provide collaborators with access to our key intellectual property rights.
Our success depends in part on the efforts of our current and possible future collaborators, who will likely have substantial control and discretion over the continued development and commercialization of drug candidates that are the subject of our collaborations.
Our current collaborators and future collaborators will have significant discretion in determining the effort and amount of resources that they dedicate to our collaborations. Our collaborators may determine not to proceed with clinical development or commercialization of a particular drug candidate for a number of reasons that are beyond our control, even under circumstances where we might have continued such a program, currently including PRS-060 and PRS-344. In addition, our rights to receive milestone payments and royalties from our collaborators will depend in part on our collaborators’ abilities to establish the safety and efficacy of our drug candidates, obtain regulatory approvals and achieve market acceptance of products developed from our drug candidates. We may also depend on our collaborators to manufacture clinical scale quantities of some of our drug candidates and, possibly, for commercial scale manufacture, distribution and sales. Our collaborators may not be successful in manufacturing our drug candidates or successfully commercializing them.
We face additional risks in connection with our existing and future collaborations, including the following:
| |
• | our collaborators may develop and commercialize, either alone or with others, products that are similar to or competitive with the products that are the subject of the collaboration with us; |
| |
• | our collaborators may underfund, not commit sufficient resources to, or conduct in an unsatisfactory manner the development, testing, marketing, distribution or sale of our drug candidates; |
| |
• | our collaborators may not properly maintain or defend our intellectual property rights or they may utilize our proprietary information in such a way as to invite litigation that could jeopardize or potentially invalidate our intellectual property or proprietary information or expose us to potential liability; |
| |
• | our collaborators may encounter conflicts of interest, changes in business strategy or other business issues that could adversely affect their willingness or ability to fulfill their obligations to us (for example, pharmaceutical and biotechnology companies historically have re-evaluated their priorities following mergers and consolidations, which have been common in recent years in these industries); |
| |
• | disputes may arise between us and our collaborators delaying or terminating the research, development, manufacture or commercialization of our drug candidates, resulting in significant litigation or arbitration that could be time-consuming and expensive, or causing collaborators to act in their own self-interest and not in the interest of our stockholders; |
| |
• | we might not have the financial or human resources to meet our obligations or take advantage of our rights under the terms of our existing and future collaborations; and |
| |
• | our existing collaborators may exercise their respective rights to terminate without cause their collaborations with us, in which event, we might not be able to complete development and commercialization of our drug candidates on our own. |
Our collaborative relationships may not produce the financial benefits that we are anticipating, which could cause our business to suffer.
Part of our strategy is to partner with, or out-license selective products to, other pharmaceutical companies in order to mitigate the cost of developing a drug through clinical trials to commercialization. Our ASKA Option Agreement is an example of this strategy. Following an analysis period after the completion of the planned phase 2a study we conducted, ASKA may exercise its option to obtain an exclusive license to develop and commercialize PRS-080 in Japan, South Korea and certain other Asian markets. Should ASKA exercise the option, we would be eligible for more than $80 million in combined option exercise fee and milestones associated with development and commercialization of PRS-080 in the first indication in Japan. We may receive further development milestones in additional indications, as well as in other countries within the ASKA territory. Even if we successfully complete our phase 2a study and such study yields favorable results, we cannot guarantee that ASKA will exercise its option with respect to PRS-080. If ASKA chooses not to exercise its option, we may or may not continue to develop PRS-080 on our own, but the post-option exercise developmental and sales milestone payments described in the ASKA Option Agreement, plus additional royalty revenues, will never be realized. If our collaboration with ASKA or other similar partners is not successful, and if we cannot earn revenue from collaborative arrangements such as this agreement, our future revenues and business will be harmed.
We may not receive any further milestone, royalty or license payments under our current collaborations.
Although we have received upfront, milestone and other payments to date under our current drug development collaborations, we may not receive any royalty payments or additional license and milestone fees under such agreements. In general, our receipt of milestone, royalty or license payments depends on many factors, including whether our collaborators want and are able to continue to pursue potential drug candidates, intellectual property issues, unforeseen complications in the development or commercialization process, and the ultimate commercial success of the drugs.
Risks Related to the Commercialization of our Drug Candidates
Even if we receive regulatory approval for any of our drug candidates, we will be subject to ongoing regulatory obligations and review. Maintaining compliance with ongoing regulatory requirements may result in significant additional expense to us, and any failure to maintain such compliance could subject us to penalties and cause our business to suffer.
Any regulatory approvals that we receive for our drug candidates may be subject to limitations on the approved indicated uses for which the products may be marketed, or contain requirements for potentially costly post-marketing testing, including phase 4 studies. In addition, if the FDA, EMA or a comparable foreign regulatory authority approves any of our drug candidates, the manufacturing processes, labeling, packaging, distribution, AE reporting, storage, advertising, promotion and record keeping
for the products will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMP. Later discovery of previously unknown problems with a product, including AEs of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| |
• | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| |
• | fines or warning letters; |
| |
• | refusal of the FDA or other applicable regulatory authority to approve pending applications or supplements to approved applications; |
| |
• | product seizure or detention, recalls, or refusal to permit the import or export of products; and |
| |
• | consent decrees, injunctions or the imposition of civil or criminal penalties. |
In addition, regulatory authorities’ policies (such as those of the FDA or EMA) may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our drug candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are otherwise not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
Our commercial success depends upon attaining significant market acceptance of our drug candidates, if approved, among physicians, patients, healthcare payors and other members of the medical community.
Even if we obtain regulatory approval for our drug candidates, the products may not gain market acceptance among physicians, health care payors, patients and other members of the medical community, which is critical to commercial success. Market acceptance of any drug candidate for which we receive approval depends on a number of factors, including:
| |
• | perceptions by the medical community, physicians, and patients, regarding the safety and effectiveness of our products; |
| |
• | the size of the market for such drug candidate, based on the size of the patient subsets that we are targeting, in the territories for which we gain regulatory approval and have commercial rights; |
| |
• | the potential and perceived advantages of the drug candidate over alternative treatments; |
| |
• | the safety of the drug candidate as demonstrated through broad commercial distribution; |
| |
• | the availability of adequate reimbursement and pricing for our products from governmental health programs and other third-party payors; |
| |
• | relative convenience and ease of administration; |
| |
• | cost-effectiveness of our product relative to competing products; |
| |
• | the prevalence and severity of adverse effects; and |
| |
• | the effectiveness of sales, marketing and distribution efforts by us and our licensees and distributors, if any. |
If our drug candidates are approved but fail to achieve an adequate level of acceptance by key market participants, we will not be able to generate significant revenues, and we may not become or remain profitable, which may require us to seek additional financing.
Our product candidates have never been manufactured on a commercial scale, and there are risks associated with scaling up manufacturing to commercial scale.
Our product candidates have never been manufactured on a commercial scale, and there are risks associated with scaling up manufacturing to commercial scale including, among others, cost overruns, potential problems with process scale-up, process reproducibility, stability issues, lot consistency and timely availability of raw materials. There is no assurance that our manufacturers will be successful in establishing a larger-scale commercial manufacturing process for PRS-060, PRS-343, PRS-080 or other product candidates that achieves our objectives for manufacturing capacity and cost of goods. Even if we could otherwise obtain regulatory approval for any product candidate, there is no assurance that our manufacturers will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product or to meet potential future demand. If our manufacturers are unable to produce sufficient quantities of the approved product for commercialization, our commercialization efforts would be impaired, which would have an adverse effect on our business, financial condition, results of operations and growth prospects.
Reimbursement may be limited or unavailable in certain market segments for our drug candidates, which could make it difficult for us to sell on a profitable basis any products for which we obtain marketing approvals.
There is significant uncertainty related to the third-party coverage and reimbursement of newly approved drugs. Market acceptance and successful commercialization of any of our drug candidates that obtain regulatory approval in domestic or international markets will depend significantly on the availability of adequate coverage and reimbursement from governmental authorities, private health insurers and other third-party payors for any of our drug candidates, and may be affected by existing and future healthcare reform measures.
Pricing and reimbursement for any of our drug candidates that obtain regulatory approval is uncertain. Government authorities, private health insurers and other third-party payors decide which drugs they will cover and establish reimbursement levels for them and obtaining coverage and reimbursement approval for a product from any such third-party payors is a time consuming and costly process. Third-party payers are also increasingly challenging the effectiveness of and prices charged for medical products and services. As a result, any denial of private or government payor coverage or inadequate reimbursement for our drug candidates, if any are commercialized, could harm our business and reduce our prospects for generating revenue.
Further, there have been, and may continue to be, legislative and regulatory proposals at the US federal and state levels and in foreign jurisdictions directed at broadening the availability and containing or lowering the cost of healthcare. Existing legislation aimed at patient affordability in the United States may be repealed or replaced. The continuing efforts of the government, insurance companies, managed care organizations and other third-party payors to contain or reduce costs of healthcare may adversely affect our ability to set prices for our products that would allow us to achieve or sustain profitability. In addition, governments may impose price controls on any of our products that obtain marketing approval, which may adversely affect our future profitability.
In some foreign countries, particularly in the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can be a long and expensive process after the receipt of marketing approval for a drug candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct additional clinical trials that compare the cost-effectiveness of our drug candidates to other available therapies. If reimbursement of our drug candidates is unavailable or limited in scope or amount in a particular country, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability for sales of any of our drug candidates that are approved for marketing in that country.
We have no experience selling, marketing or distributing products and currently have no internal marketing and sales force. If we are unable to establish effective marketing and sales capabilities or enter into agreements with third parties to market and sell our drug candidates, we may not be able to effectively market and sell our drug candidates, if approved, or generate product revenues.
We currently have no sales, marketing or distribution capabilities and there can be no assurance that we will be able to market and sell our products in the United States or overseas. In order to commercialize any drug candidates, we must build on a territory-by-territory basis marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. Therefore, with respect to the commercialization of all or certain of our drug candidates, we may choose to collaborate, either globally or on a territory-by-territory basis, with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. If so, our success will depend, in part, on our ability to enter into and maintain collaborative relationships for such capabilities, such collaborators’ strategic interest in the products under development and such collaborators’ ability to successfully market and sell any such products.
If we are unable to enter into such arrangements when needed on acceptable terms or at all, we may not be able to successfully commercialize any of our drug candidates that receive regulatory approval, or any such commercialization may experience delays or limitations. Further, to the extent that we depend on third parties for marketing and distribution, any revenues we receive will depend upon the efforts of such third parties, and there can be no assurance that such efforts will be successful.
To the extent that we decide not to, or are unable to, enter into collaborative arrangements with respect to the sales and marketing of our products, we may in the future need to establish an internal sales and marketing team with technical expertise and supporting distribution capabilities to commercialize our drug candidates, which could be expensive and time consuming and which would require significant attention of our executive officers to manage. Further, we may not have sufficient resources to allocate to the sales and marketing of our products.
Any failure or delay in the development of sales, marketing and distribution capabilities, through collaboration with one or more third parties or through internal efforts, would adversely impact the commercialization of any of our products that we obtain approval to market. As a result, our future product revenue will suffer and we may incur significant additional losses.
Risks Related to Our Intellectual Property
If we breach any of the agreements under which we license from third parties the intellectual property rights or commercialization rights to our drug candidates, particularly our license agreements with TUM, Enumeral and Kelun, we could lose license rights that are important to our business and our operations could be materially harmed.
We in-license significant intellectual property related to our Anticalin platforms from TUM. Under the terms of the TUM License, TUM assigns to us certain materials and records resulting from the research. We retain rights to inventions made by our employees, and TUM assigns to us all inventions made under the agreement jointly by our employees and TUM personnel, provided that our employees have made a certain inventive contribution. With respect to all other inventions made in the course of the research, TUM grants to us worldwide exclusive license rights under patents and patent applications claiming such inventions. TUM retains rights to practice these inventions for research and teaching purposes. We bear the costs of filing, prosecuting and maintaining the patents assigned or licensed to us under the TUM License.
As consideration for the assignments and licenses, we are obliged to pay to TUM milestone payments on development of our proprietary products claimed by patents assigned or licensed to us by TUM. We also are obliged to pay low single-digit royalties, including annual minimum royalties, on the sales of such products. Should we grant licenses or sublicenses to those patents to third parties, we are obliged to pay to TUM certain undisclosed fees as a function of out-licensing revenues in connection with those patents, or Out-License Fees, where such Out-License Fees are creditable against annual license payments to TUM. Our payment obligations are reduced by our proportionate contribution to a joint invention. Payment obligations terminate on expiration or annulment of the last patent covered by the TUM License that covers a proprietary product or is sublicensed, as applicable.
Pieris and TUM initiated discussions in the second quarter of 2018 to clarify, expand and restructure the TUM License, including the parties’ obligations under such license agreement. The parties' recent discussions relate to revised commercial terms and to re-initiating additional collaborations between faculty at TUM and Pieris. While an amended and restated license agreement has not yet been completed, we intend to enter into such an amendment. We recorded the probable expected impact of the amendment in research and development expense in 2018, which is an increase in our financial obligations associated with the TUM License of approximately $2.3 million for amounts that would be due in 2019 for 2018 and 2017 sub-licensing activities. These discussions may also lead to an increase in our collaborative research activities with TUM.
Under the PD-1 In-License with Enumeral, we in-licensed intellectual property related to an Enumeral-generated antibody against PD-1 and are granted an option to in-license up to two additional antibodies against undisclosed targets. Under the terms of the PD-1 In-License, we acquired a non-exclusive worldwide license under the applicable Enumeral patents and know-how to research, develop and commercialize fusion proteins incorporating Enumeral’s PD-1 antibody and one or more Anticalin proteins. On January 29, 2018, Enumeral filed a voluntary petition for relief under Chapter 11 of the United States Bankruptcy Code in the Bankruptcy Court. In connection with those proceedings, Enumeral transferred the intellectual property related to the PD-1 In-License to Acquisition Group, who have assumed the rights and obligations of Enumeral with respect to the PD-1 In-License.
As consideration, we are obliged to pay to Acquisition Group development and sales milestones on development of products incorporating the Enumeral antibody. We are also obliged to pay low to lower-middle single-digit royalties as a percentage of net sales depending on the amount of net sales in the applicable years. In the event that we are required to pay a license fee or royalty to any third party related to the licensed products, our royalty payment obligations to Acquisition Group are reduced by the amount of such third-party fees or payments, up to 50% of the royalty payment for each calendar year due to Acquisition Group. Payment obligations terminate on a product-by-product and country-by-country basis on the later of 10 years from the first commercial sale of a product incorporating the Enumeral antibody or the last to expire, lapse or be abandoned of a claim from the licensed Enumeral patents filed as of the effective date of the PD-1 In-License that cover the manufacture, use, offer for sale, sale or import of a product incorporating the Enumeral antibody.
In connection with our efforts to develop multispecific Anticalin-based proteins designed to engage immunomodulatory targets, during the second quarter of 2017, we entered into the Kelun Agreement. Under the Kelun Agreement, Kelun has granted to us a non-exclusive worldwide license (with the right to sublicense) under certain intellectual property owned or controlled by Kelun to research, develop, manufacture and commercialize bi- and multi- specific fusion proteins that include an antibody developed by Kelun specific for an undisclosed target and one or more Anticalin proteins.
In addition to the TUM License and the PD-1 In-License, we have other in-license agreements and may seek to enter into additional agreements with other third parties in the future granting similar license rights with respect to other potential drug candidates. If we fail to comply with any of the conditions or obligations or otherwise breach the terms of the TUM License or the PD-1 In-License, the Kelun Agreement, or any future license agreement we may enter on which our business or drug candidates are dependent, TUM, Enumeral, Kelun, or other licensors may have the right to terminate the applicable agreement in whole or in part and thereby extinguish our rights to the licensed technology and intellectual property and/or any rights we have acquired to develop and commercialize certain drug candidates, including, with respect to the TUM License and PD-1 In-License, our Anticalin drug therapies. Under the TUM License, we can terminate the licenses to any or all licensed patents upon specified advance notice to TUM. TUM may terminate the license provisions of the agreement only for cause. Termination of the TUM License does not terminate our rights in patents assigned to us but would terminate our rights to patents licensed to us under the agreement. Under the PD-1 In-License, we can terminate the agreement upon 30 days’ notice to Enumeral. Enumeral may terminate the PD-1 In-License only upon a material breach by us that is not cured. The loss of the rights licensed to us under our license agreement with TUM or Enumeral, or any future license agreement that we may enter granting us rights on which our business or drug candidates are dependent, would eliminate our ability to further develop the applicable drug candidates and would materially harm our business, prospects, financial condition and results of operations.
If our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not be able to compete effectively and our business would be harmed.
We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our technologies. Any disclosure to, or misappropriation by, third parties of our proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding any competitive advantage we may derive from the proprietary information.
The strength of patents in the biotechnology and pharmaceutical fields can be uncertain and involve complex legal and scientific questions. No consistent policy regarding the breadth of claims allowed in patents has emerged to date in the United States. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced, or that the scope of any patent rights could provide a sufficient degree of protection that could permit us to gain or keep our competitive advantage with respect to these products and technologies. For example, we cannot predict:
| |
• | the degree and range of protection any patents will afford us against competitors, including whether third parties will find ways to make, use, sell, offer to sell or import competitive products without infringing our patents; |
| |
• | if and when patents will be issued; |
| |
• | whether or not others will obtain patents claiming inventions similar to those covered by our patents and patent applications; or |
| |
• | whether we will need to initiate litigation or administrative proceedings (for example, at the USPTO, or the European Patent Office, or the EPO) in connection with patent rights, which may be costly whether we win or lose. |
As a result, the patent applications we own or license may fail to result in issued patents in the United States or in foreign countries. Third parties may challenge the validity, enforceability or scope of any issued patents we own or license or any applications that may issue as patents in the future, which may result in those patents being narrowed, invalidated or held unenforceable. Even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from developing similar products that do not fall within the scope of our patents. If the breadth or strength of protection provided by the patents we hold or pursue is threatened, our ability to commercialize any drug candidates with technology protected by those patents could be threatened. Further, if we encounter delays in our clinical trials, the period of time during which we would have patent protection for any covered drug candidates that obtain regulatory approval would be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain at the time of filing that we are the first to file any patent application related to our drug candidates.
While patent term extensions under the Hatch-Waxman Act in the United States and under supplementary protection certificates in Europe may be available to extend our patent exclusivity for our drug candidates, the applicable patents may not meet the specified conditions for eligibility for any such term extension and, even if eligible, we may not be able to obtain any such term extension. Further, because filing, prosecuting, defending and enforcing patents in multiple jurisdictions can be expensive, we may elect to pursue patent protection relating to our drug candidates in only certain jurisdictions. As a result, competitors would be permitted to use our technologies in jurisdictions where we have not obtained patent protection to develop their own products, any of which could compete with our drug candidates.
In addition to the protection afforded by patents, we seek to rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our discovery platform and drug development processes that involve proprietary know-how, information or technology that is not covered by patents or not amenable to patent protection. Although we require all of our employees and certain consultants and advisors to assign inventions to us, and require all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, our trade secrets and other proprietary information may be disclosed or competitors may otherwise gain access to such information or independently develop substantially equivalent information. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant difficulty in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the trade secrets and other intellectual property related to our technologies to third parties, we may not be able to establish or maintain the competitive advantage that we believe is provided by such intellectual property, which could adversely affect our market position and business and operational results.
Claims that we infringe the intellectual property rights of others may prevent or delay our drug discovery and development efforts.
Our research, development and commercialization activities, as well as any drug candidates or products resulting from those activities, may infringe or be accused of infringing a patent or other form of intellectual property under which we do not hold a license or other rights. Third parties may assert that we are employing their proprietary technology without authorization.
There may be third-party patents of which we are currently unaware with claims that cover the use or manufacture of our drug candidates or the practice of our related methods. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our drug candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our drug candidates infringes upon one or more claims of these patents. If our activities or drug candidates infringe the patents or other intellectual property rights of third parties, the holders of such intellectual property rights may be able to block our ability to commercialize such drug candidates or practice our methods unless we obtain a license under the intellectual property rights or until any applicable patents expire or are determined to be invalid or unenforceable.
Defense of any intellectual property infringement claims against us, regardless of their merit, would involve substantial litigation expense and would be a significant diversion of resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, obtain one or more licenses from third parties, limit our business to avoid the infringing activities, pay royalties and/or redesign our infringing drug candidates or alter related formulations, processes, methods or other technologies, any or all of which may be impossible or require substantial time and monetary expenditure. Further, if we were to seek a license from the third-party holder of any applicable intellectual property rights, we may not be able to obtain the applicable license rights when needed or on reasonable terms, or at all. Some of our competitors may be able to sustain the costs of complex patent litigation or proceeding more effectively than us due to their substantially greater resources. The occurrence of any of the above events could prevent us from continuing to develop and commercialize one or more of our drug candidates and our business could materially suffer.
We may desire to, or be forced to, seek additional licenses to use intellectual property owned by third parties, and such licenses may not be available on commercially reasonable terms or at all.
Third parties may also hold intellectual property, including patent rights that are important or necessary to the development of our drug candidates, in which case we would need to obtain a license from that third party or develop a different formulation of the product that does not infringe upon the applicable intellectual property, which may not be possible. Additionally, we may identify drug candidates that we believe are promising and whose development and other intellectual property rights are held by third parties. In such a case, we may desire to seek a license to pursue the development of those drug candidates. Any license that we may desire to obtain or that we may be forced to pursue may not be available when needed on commercially reasonable terms or at all. Any inability to secure a license that we need or desire could have a material adverse effect on our business, financial condition and prospects.
The patent protection covering some of our drug candidates may be dependent on third parties, who may not effectively maintain that protection.
While we expect the right to fully prosecute any patents covering drug candidates we may in-license from third-party owners, there may be instances when the prosecution and maintenance of issued patents and pending patent applications that cover our drug candidates remain controlled by our licensors. Similarly, some of our future licensing partners may retain the right, or may seek the rights, to prosecute patents covering the drug candidates we license to them and we may grant such rights to those partners for business reasons. If such third parties fail to appropriately maintain that patent protection, we may not be able to prevent competitors from developing and selling competing products or practicing competing methods and our ability to generate revenue from any commercialization of the affected drug candidates may suffer.
Certain technologies and patents have been developed with partners and we may face restrictions on this jointly developed intellectual property.
We have entered into agreements with a number of commercial partners, including university partners, which cover intellectual property. We have, in some cases individually and in other cases along with our partners, filed for patent protection for a number of technologies developed under these agreements and may in the future file for further intellectual property protection and/or seek to commercialize such technologies. Under some of these agreements, certain intellectual property developed by us and the relevant partner may be subject to joint ownership by us and the partner and our commercial use of such intellectual property may be restricted, or may require written consent from, or a separate agreement with, the partner. In other cases, we may not have any rights to use intellectual property solely developed and owned by the partner. If we cannot obtain commercial use rights for such jointly owned intellectual property or partner-owned intellectual property, our future product development and commercialization plans may be adversely affected.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our current or potential licensors. To attempt to stop infringement or unauthorized use, we may need to enforce one or more of our patents, which can be expensive and time-consuming and distract management. If we pursue any litigation, a court may decide that a patent of ours or any of our licensors’ is not valid or is unenforceable or may refuse to stop the other party from using the relevant technology on the grounds that our patents do not cover the technology in question. Further, the legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, which could reduce the likelihood of success of, or the amount of damages that could be awarded resulting from, any infringement proceeding we pursue in any such jurisdiction. An adverse result in any infringement litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not issuing, which could limit our ability to exclude competitors from directly competing with us in those jurisdictions.
Interference proceedings provoked by third parties or brought by the USPTO or at its foreign counterparts (such as the EPO) to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to use it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms, or at all.
Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees.
If we are unsuccessful in obtaining or maintaining patent protection for intellectual property in development, our business and competitive position would be harmed.
We are seeking patent protection of our technology and for our drug candidates. Patent prosecution is a challenging process and is not assured of success. If we are unable to secure patent protection for our technology and drug candidates, our business may be adversely impacted.
In addition, issued patents and pending applications require regular maintenance. Failure to maintain our portfolio may result in loss of rights that may adversely impact our intellectual property rights, for example by rendering issued patents unenforceable or by prematurely terminating pending applications.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for our Anticalin-brand technology and some of our drug candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We currently, and expect in the future to continue to, seek to protect these trade secrets, in part by entering into confidentiality agreements with parties who have access to them, such as our employees, collaborators, CMOs, consultants, advisors, investigators and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for any such disclosure. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they disclose the trade secrets, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
If we fail to protect our trademark rights, competitors may be able to take advantage of our goodwill, which would weaken our competitive position, reduce our revenues and increase our costs.
We believe that the protection of our trademark rights is an important factor in product recognition, maintaining goodwill, and maintaining or increasing market share. We may expend substantial cost and effort in an attempt to register, maintain and enforce our trademark rights. If we do not adequately protect our rights in our trademarks from infringement, any goodwill that we have developed in those trademarks could be lost or impaired.
Third parties may claim that the sale or promotion of our products, when and if we have any, may infringe on the trademark rights of others. Trademark infringement problems occur frequently in connection with the sale and marketing of pharmaceutical products. If we become involved in any dispute regarding our trademark rights, regardless of whether we prevail, we could be required to engage in costly, distracting and time-consuming litigation that could harm our business. If the trademarks we use are found to infringe upon the trademark of another company, we could be liable for damages and be forced to stop using those trademarks, and as result, we could lose all the goodwill that has been developed in those trademarks.
The future growth of our business may expose our intellectual property to a high risk of counterfeiting or unauthorized use.
As part of our business strategy, we intend to license our Anticalin technology and sell our potential products, if any, in many different countries. As a result, we may do business with third parties in countries where intellectual property rights have been or are routinely disregarded, and the future growth of our business may expose our intellectual property to a high risk of counterfeiting or unauthorized use. Although we attempt to obtain broad international intellectual property rights for our Anticalin technology and proteins, we cannot guarantee that such rights, to the extent we can obtain them, will be enforceable in a timely fashion or at all in any particular country or jurisdiction, or that if enforced, will offer us adequate commercial protection or adequate redress for any harm suffered. Counterfeiting or unauthorized use of our technologies or products may also expose our business to harm for which no adequate monetary redress exists, and to the extent we are unable to stop such use, may cause us to lose rights with respect to intellectual property that is crucial to our business. Any such misuse of our intellectual property may have a substantial negative impact on our business and revenues and may cause our business to fail.
Risks Related to our Employees
If we are not able to attract and retain highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive biotechnology and pharmaceuticals industries depends upon our ability to attract and retain highly qualified personnel. We are highly dependent on our management, scientific and medical personnel, especially Stephen S. Yoder, our Chief Executive Officer and President, whose services are critical to the successful implementation of our drug candidate development, our business development and partnerships, and our regulatory and commercialization strategies. Further, as our approach is built upon the drug discovery and development experience of our drug development team, which we believe is a significant contributor to our competitive advantage, we are dependent on the maintenance and growth of that team with qualified members containing high levels of expertise in specific scientific fields. We may in the future hire additional employees for research and development or general and administrative activities.
We are not aware of any present intention of any of our executive officers or other members of our senior management team to leave our company, but our industry tends to experience a high rate of turnover of management personnel and our employees are generally able to terminate their relationships with us on short notice. Pursuant to German employment law, our employment arrangements with employees of Pieris GmbH are governed by employment contracts, which provide certain defined terms for either party to terminate the employment relationship.
The loss of the services of any of our executive officers, in particular Mr. Yoder, or other key employees and our inability to find suitable replacements could potentially harm our business, financial condition and prospects. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior and mid-level managers as well as junior and mid-level scientific and medical personnel.
Moreover, there is intense competition for a limited number of qualified personnel among biopharmaceutical, biotechnology, pharmaceutical and other related businesses. Many of the other companies against which we compete for qualified personnel have greater financial and other resources, different risk profiles, longer histories in the industry and greater ability to provide valuable cash or stock incentives to potential recruits than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than what we are able to offer as an early stage company. If we are unable to continue to attract and retain high quality personnel, the rate and success at which we can develop and commercialize our drug candidates will be limited.
We may be subject to labor claims brought by our employees against us.
In the United States, an employment relationship with no specified duration is presumed to be employment “at-will” and the employer or employee may terminate the employment relationship at any time, with or without cause, except for public policy reasons including discrimination, participating in union activity, or refusing to carry out an activity that violates the law.
In contrast, in Germany, there is no analogous doctrine of “employment at will.” By law, German employees must have written employment contracts that reflect the key aspects of the employment relationship. Our relations between German employers and employees are extensively regulated under German labor and employment laws and regulations. German employees have special protection against dismissals provided the employee has been employed by a company for more than six months and such company employs more than 10 employees.
German employment termination law is regulated by various codes, in particular the Kündigungsschutzgesetz, or the German Termination Protection Act, and is intended to give the employee maximum protection against unfair dismissal, including among other things:
| |
• | the employer must observe the applicable notice period, which is ordinarily determined by law (between four weeks and seven months, depending upon the length of employment), if a longer period is not otherwise agreed by the parties, and has to deliver a written notice of termination to the employee; |
| |
• | for companies with more than 10 employees, the German Termination Protection Act generally restricts termination of employment if the employee has been employed for more than six months, wherein the employee may be terminated only for a particular reason, including certain behavioral or personal reasons relating to the employee or certain developments relating to the business of the employer, such as a business restructuring which reduces the number of employee positions; |
| |
• | special termination protection against unlawful dismissal applies to several other groups of employees, such as an employee that is an officially acknowledged handicapped person, an employee who was appointed as a company’s data protection officer or as a member of the works council of a company, if any, an employee on three years’ maternity leave or a pregnant employee; in these cases, approval of various German authorities is required prior to termination but usually very difficult to obtain; and |
| |
• | if a company engages in a mass layoff, which is deemed to occur when the employer intends to dismiss a large percentage of its employees during a one-month period, prior written notification to the German employment office is required. |
In this regard, if we downsize for any reason and fail to adhere to the complex requirements articulated by the employee protection law, we could face legal actions brought by affected employees or former employees, and, as a result, we may incur operational or financial losses and the attention of our executive officers may be distracted from managing our business.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property or claiming ownership of what we regard as our own intellectual property.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employers. Litigation may be necessary to defend against any such claims.
In addition, while it is our policy to require our employees and contractors, who may be involved in the development of intellectual property, to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact contributes to the development of intellectual property that we regard as our own. Further, the terms of such assignment agreements may be breached and we may not be able to successfully enforce their terms, which may force us to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of intellectual property rights we may regard and treat as our own.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could cause our business to suffer.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA or EMA regulations, provide accurate information to the FDA or EMA, comply with manufacturing standards we have established, comply with federal, state and international healthcare fraud and abuse laws and regulations as they may become applicable to our operations, report financial information or data accurately or disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions and procedures we currently take or may establish in the future as our operations and employee base expand to detect and prevent this type of activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure by our employees to comply with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
Certain of our employees and their inventions are subject to German law.
Many of our employees work in Germany and are subject to German employment law. Ideas, developments, discoveries and inventions made by such employees and consultants are subject to the provisions of the Gesetz über Arbeitnehmererfindungen, or the German Act on Employees’ Inventions, which regulates the ownership of, and compensation for, inventions made by employees. We face the risk that disputes can occur between us and such employees or ex-employees pertaining to alleged non-adherence to the provisions of this act that may be costly to defend and take up our management’s time and efforts whether we prevail or fail in such dispute. In addition, under the German Act on Employees’ Inventions, certain employees retained rights to patents they invented or co-invented prior to 2009. Although most of these employees have subsequently assigned their interest in these patents to us, there is a risk that the compensation we provide to them may be deemed insufficient and we may be required under German law to increase the compensation due to such employees for the use of the patents. In those cases where employees have not assigned their interests to us, we may need to pay compensation for the use of those patents. If we are required to pay additional compensation or face other disputes under the German Act on Employees’ Inventions, our results of operations could be adversely affected.
Risks Related to the Ownership of our Common Stock
Our share price is expected to be volatile and may be influenced by numerous factors, some of which are beyond our control.
Market prices for shares of biotechnology companies such as ours are often volatile, and the quoted price of our common stock is therefore likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this Annual Report on Form 10-K, these factors include:
| |
• | the drug candidates we seek to pursue, and our ability to obtain rights to develop, commercialize and market those drug candidates; |
| |
• | our decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial; |
| |
• | actual or anticipated adverse results or delays in our clinical trials; |
| |
• | our failure to commercialize our drug candidates, if approved; |
| |
• | unanticipated serious safety concerns related to the use of any of our drug candidates; |
| |
• | adverse regulatory decisions; |
| |
• | additions or departures of key scientific or management personnel; |
| |
• | changes in laws or regulations applicable to our drug candidates, including without limitation clinical trial requirements for approvals; |
| |
• | the perception of the pharmaceutical and biotechnology industry by the public, legislatures, regulators and the investment community; |
| |
• | disputes or other developments relating to patents and other proprietary rights and our ability to obtain patent protection for our drug candidates; |
| |
• | significant lawsuits, including patent and stockholder class action litigation; |
| |
• | our dependence on third parties, including CROs and CMOs as well as our current and potential partners that produce companion diagnostic products; |
| |
• | failure to meet or exceed any financial guidance or expectations regarding development milestones that we may provide to the public; |
| |
• | actual or anticipated variations in quarterly operating results; |
| |
• | failure to meet or exceed the estimates and projections of the investment community; |
| |
• | overall performance of the equity markets and other factors that may be unrelated to our operating performance or the operating performance of our competitors, including changes in market valuations of similar companies; |
| |
• | conditions or trends in the biotechnology and biopharmaceutical industries; |
| |
• | introduction of new products offered by us or our competitors; |
| |
• | announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors; |
| |
• | our ability to maintain an adequate rate of growth and manage such growth; |
| |
• | issuances of debt or equity securities; |
| |
• | sales of our common stock by us or our stockholders in the future, or the perception that such sales could occur; |
| |
• | trading volume of our common stock; |
| |
• | ineffectiveness of our internal control over financial reporting or disclosure controls and procedures; |
| |
• | general political and economic conditions; |
| |
• | effects of natural or man-made catastrophic events; and |
| |
• | other events or factors, many of which are beyond our control. |
In addition, the stock market in general, and the stocks of biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In addition, other biotechnology companies or our competitors’ programs could have positive or negative results that impact their stock prices and their results or stock fluctuations could have a positive or negative impact on our stock price regardless of whether such impact is direct or not. The realization of any of the above risks or any of a broad range of other risks, including those described in these “Risk Factors,” could have a dramatic and material adverse impact on the market price of our common stock.
We have broad discretion in how we use our cash, cash equivalents and marketable securities, including the net proceeds from our collaborations, public and private securities offerings, and may not use these financial resources effectively, which could affect our results of operations and cause our stock price to decline.
Our management has considerable discretion in the application of our cash, cash equivalents and marketable securities, including the fees and milestone payments from our collaborations and the net proceeds of our securities offerings. We intend to use the cash, cash equivalents and marketable securities to advance our product candidates and for working capital and other general corporate purposes, which will include the hiring of additional personnel and capital expenditures. As a result, investors will be relying upon management's judgment with only limited information about our specific intentions for the use of the cash, cash equivalents and marketable securities. We may use the cash, cash equivalents and marketable securities for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the financial resources from our collaborations and securities offerings in a manner that does not produce income or that loses value.
If securities or industry analysts do not publish, or cease publishing, research or publish inaccurate or unfavorable research about our business or our market, or if they change their recommendations regarding our stock adversely, our stock price and any trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. If only a few securities or industry analysts commence coverage of our company, the trading price for our stock would likely be negatively affected and there can be no assurance that analysts will provide favorable coverage. If securities or industry analysts who initiate coverage downgrade our stock or publish inaccurate or unfavorable research about our business or our market, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and any trading volume to decline. As of December 31, 2018, we had five analysts covering our stock. We lack the potential benefits that coverage by additional analysts may provide.
We have a material weakness in our internal controls over financial reporting, and we have had other materials weaknesses in the past. If we fail to maintain proper and effective internal controls, our ability to produce accurate and timely financial statements could be impaired, which could harm our operating results, our ability to operate our business and investors’ views of us.
We are required to comply with Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act, subject to certain exceptions. Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an annual review and evaluation of their internal controls and to obtain attestations of the effectiveness of internal controls by independent auditors. However, as discussed in detail below, as an emerging growth company, we are not required to obtain an auditor attestation.
Under the JOBS Act, issuers that qualify as “emerging growth companies” under the JOBS Act will not be required to provide an auditor’s attestation report on internal controls for so long as the issuer qualifies as an emerging growth company. We currently qualify as an emerging growth company under the JOBS Act, and we have chosen not to provide an auditor’s attestation report on internal controls. However, if we cannot favorably assess the effectiveness of our internal control over financial reporting, or if we require an attestation report from our independent registered public accounting firm in the future and that firm is unable to provide an unqualified attestation report on the effectiveness of our internal controls over financial reporting, investor confidence and, in turn, our stock price could be materially adversely affected.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Our failure to remediate our material weakness in internal controls and thereafter to maintain the effectiveness of our internal controls in accordance with the requirements of the Sarbanes-Oxley Act could have a material adverse effect on the tradability of our common stock, which in turn would negatively impact our business. We could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on the price of our common stock. In addition, if our efforts to comply with new or changed laws, regulations, and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
As reported in our annual report on Form 10-K for the year ended December 31, 2017, we concluded that we had a material weakness relating to the financial statement close process due to the combination of deficiencies. The deficiencies resulted from two separate errors that were not identified by management; one related to the classification of certain operating expenses and one related to the reporting of foreign currency re-measurements on investments. As of December 31, 2018, we remediated this weakness by implementing corrective measures noted in Item 9A. in Part II of this Annual Report on Form 10-K. We cannot assure you that additional material weaknesses or significant deficiencies in our internal control over financial reporting will not be identified in the future.
As reported in this Annual Report on Form 10-K, we concluded that we had a material weakness relating to our income tax provision process, including the evaluation of any changes resulting from the recently enacted TCJA. The material weakness created a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements may not be prevented or detected on a timely basis. The material weakness did not result in any misstatement or correction in the provision for income taxes prior to the issuance of the 2018 consolidated financial statements included in this Form 10-K.
Shares of our common stock that have not been registered under federal securities laws are subject to resale restrictions imposed by Rule 144 of the Securities Act, including those set forth in Rule 144(i) which apply to a former “shell company.”
Prior to the closing of the Acquisition, we were deemed a “shell company” under applicable SEC rules and regulations because we had no or nominal operations and either no or nominal assets, assets consisting solely of cash and cash equivalents, or assets consisting of any amount of cash and cash equivalents and nominal other assets. Pursuant to Rule 144 promulgated under the Securities Act, sales of the securities of a former shell company, such as us, under that rule are not permitted unless at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months, other than current reports on Form 8-K. Additionally, our previous status as a shell company could also limit our use of our securities to pay for any acquisitions we may seek to pursue in the future. The lack of liquidity of our securities as a result of the inability to sell under Rule 144 for a longer period of time than a non-former shell company could cause the market price of our securities to decline.
If we issue additional shares of our capital stock in the future, our existing stockholders will be diluted.
Our Amended and Restated Articles of Incorporation authorize the issuance of up to 300,000,000 shares of our common stock and up to 10,000,000 shares of preferred stock with the terms, limitations, voting rights, relative rights and preferences and variations of each series that our Board of Directors may determine from time to time. Possible business and financial uses for our authorized capital stock include, without limitation, equity financing, future stock splits, acquiring other companies, businesses or products in exchange for shares of our capital stock, issuing shares of our capital stock to partners or other collaborators in connection with strategic alliances, attracting and retaining employees by the issuance of additional securities under our equity compensation plan, or other transactions and corporate purposes that our Board of Directors deems are in the interests of our company. Additionally, issuances of shares of our capital stock could have the effect of delaying or preventing changes in control or our management. Any future issuances of shares of our capital stock may not be made on favorable terms or at all, they may have rights, preferences and privileges that are superior to those of our common stock and may have an adverse effect on our business or the trading price of our common stock. The issuance of any additional shares of our common
stock will reduce the book value per share and may contribute to a reduction in the market price of the outstanding shares of our common stock. Additionally, any such issuance will reduce the proportionate ownership and voting power of all of our current stockholders.
Sales of a substantial number of shares of our common stock in the public market, or the perception that such sales could occur, could cause our stock price to fall.
If our existing stockholders sell, or indicate an intention to sell, substantial amounts of our common stock in the public market, the trading price of our common stock could decline. As of December 31, 2018, a total of 54,151,219 shares of our common stock were outstanding. Any sales of those shares or any perception in the market that such sales may occur could cause the trading price of our common stock to decline.
In addition, shares of our common stock that are either subject to outstanding options or reserved for future issuance under our equity incentive plan will be eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules. If these additional shares of common stock are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
The resale of shares covered by our effective resale registration statement could adversely affect the market price of our common stock in the public market, which result would in turn negatively affect our ability to raise additional equity capital.
The sale, or availability for sale, of our common stock in the public market may adversely affect the prevailing market price of our common stock and may impair our ability to raise additional equity capital. Pursuant to registration statements filed with the SEC, we previously registered for resale (i) 27,321,870 shares of our common stock, which represents all of the shares of our common stock issued and sold in our private placement consummated in December 2014, shares of our common stock issued to former stockholders of Pieris GmbH in connection with the closing of the Acquisition, and shares of common stock issuable upon exercise of common stock purchase warrants issued in connection with the closings of the private placement in December 2014; (ii) 13,102,084 shares of common stock consisting of (w) 3,225,804 shares of our common stock issued and outstanding at the time of filing such resale registration statement, (x) 4,963,000 shares of common stock issuable upon the conversion of 4,963 shares of our Series A Convertible Preferred Stock, par value $0.001 per share, and (y) 4,913,280 shares of common stock issuable upon exercise of common stock purchase warrants, which represents all of the securities issued and sold in our private placement consummated in June 2016; and (iii) 15,250,634 shares of common stock consisting of (x) 15,250,634 shares of common stock issued and outstanding at the time of filing such resale registration statement and (y) 542,360 shares of common stock issuable upon exercise of common stock purchase warrants, which represents securities issued and sold in our private placement consummated in December 2014, shares of our common stock issued to former stockholders of Pieris GmbH in connection with the closing of the Acquisition and shares of common stock issuable upon exercise of common stock purchase warrants issued in connection with the closings of the private placement in December 2014. The resale registration statement permits the resale of these shares at any time without restriction. The resale of a substantial number of shares of our common stock in the public market could adversely affect the market price for our common stock and make it more difficult for investors to sell shares of our common stock at times and prices that investors feel are appropriate. Furthermore, because there are a large number of shares registered pursuant to the resale registration statement, we may continue to offer shares covered by the resale registration statement for a significant period of time, the precise duration of which cannot be predicted. Accordingly, the adverse market and price pressures resulting from an offering pursuant to the resale registration statement may continue for an extended period of time and continued negative pressure on the market price of our common stock could have a material adverse effect on our ability to raise additional equity capital.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to our equity incentive plans or otherwise, could result in dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
Even after giving effect to the funds raised in the past, we expect that significant additional capital will be needed in the future to continue our planned operations. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner, in which we may determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors in a prior transaction may be materially diluted. Additionally, new investors could gain rights, preferences and privileges senior to those of existing holders of our common stock. Further, any future sales of our common stock by us or resales of our common stock by our existing stockholders could cause the market price of our common stock to decline.
Pursuant to our 2018 Employee, Director and Consultant Equity Incentive Plan, or the Pieris 2018 Plan, we are authorized to grant equity awards to our employees, directors and consultants for up to an aggregate of 3,000,000 shares of our common
stock reserved for issuance pursuant to the Pieris 2018 Plan, plus an additional 6,975,000 shares granted under the 2016 Plan and 2014 Plan, including shares that expired or were canceled on or after July 24, 2018 under these plans and become available for grant under the Pieris 2018 Plan. As of December 31, 2018, we have granted options to purchase approximately 6.9 million shares of our common stock. Pursuant to our 2018 Employee Stock Purchase Plan, we are authorized to sell 500,000 shares to our employees. Any future grants of options, warrants or other securities exercisable or convertible into our common stock, or the exercise or conversion of such shares, and any sales of such shares in the market, could have an adverse effect on the market price of our common stock.
Anti-takeover provisions in our organizational documents could delay or prevent a change of control.
Certain provisions of our Amended and Restated Articles of Incorporation and Amended and Restated Bylaws may have an anti-takeover effect and may delay, defer or prevent a merger, acquisition, tender offer, takeover attempt or other change of control transaction that a stockholder might consider to be in its interests, including attempts that might result in a premium over the market price for the shares held by our stockholders.
These provisions provide, among other things:
| |
• | a classified Board of Directors with staggered three-year terms; |
| |
• | the ability of our Board of Directors to issue one or more series of preferred stock with voting or other rights or preferences that could have the effect of impeding the success of an attempt to acquire us or otherwise effect a change of control; |
| |
• | advance notice for nominations of directors by stockholders and for stockholders to include matters to be considered at stockholder meetings; |
| |
• | certain limitations on convening special stockholder meetings and the prohibition of stockholder action by written consent; and |
| |
• | directors may only be removed for cause and only by the affirmative vote of the holders of at least 80% of the voting power of all of the then-outstanding shares of our capital stock entitled to vote at an election of directors, voting together as a single class. |
These anti-takeover provisions, including those noted above, could make it more difficult for a third party to acquire us, even if the third party’s offer may be considered beneficial by many of our stockholders. As a result, our stockholders may be limited in their ability to obtain a premium for their shares. See “Description of Capital Stock.”
We may incur significant costs from class action litigation due to our expected stock volatility.
Our stock price may fluctuate for many reasons, including as a result of public announcements regarding the progress of our development efforts or the development efforts of future collaborators or competitors, the addition or departure of our key personnel, variations in our quarterly operating results and changes in market valuations of biopharmaceutical and biotechnology companies.
This risk is especially relevant to us because biopharmaceutical and biotechnology companies have experienced significant stock price volatility in recent years. When the market price of a stock has been volatile as our stock price may be, holders of that stock have occasionally brought securities class action litigation against the company that issued the stock. If any of our stockholders were to bring a lawsuit of this type against us, even if the lawsuit is without merit, could result in substantial costs defending the lawsuit and diversion of the time, attention and resources of our Board of Directors and management, which could significantly harm our profitability and reputation.
Our Amended and Restated Articles of Incorporation designates the Eighth Judicial District Court of Clark County, Nevada, as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, and therefore limit our stockholders’ ability to choose a forum for disputes with us or our directors, officers, employees or agents.
Our Amended and Restated Articles of Incorporation provide that, to the fullest extent permitted by law, and unless we consent to the selection of an alternative forum, the Eighth Judicial District Court of Clark County, Nevada shall be the sole and exclusive forum for any (i) derivative action or proceeding brought in the name or right of the corporation or on its behalf,
(ii) action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, employees or agents to the corporation or any of our stockholders, (iii) any action arising or asserting a claim arising pursuant to any provision of Chapters 78 or 92A of the Nevada Revised Statutes or any provision of our articles of incorporation or bylaws, (iv) any action to interpret, apply, enforce or determine the validity of our articles of incorporation or bylaws or (v) any action asserting a claim governed by the internal affairs doctrine. Our Amended and Restated Articles of Incorporation further provide that any person purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed, to the fullest extent permitted by law, to have notice of and consented to the foregoing provision.
We believe the choice-of-forum provision in our Amended and Restated Articles of Incorporation will help provide for the orderly, efficient and cost-effective resolution of Nevada-law issues affecting us by designating courts located in the State of Nevada (our state of incorporation) as the exclusive forum for cases involving such issues. However, this provision may limit a stockholder’s ability to bring a claim in a judicial forum that it believes to be favorable for disputes with us or our directors, officers, employees or agents, which may discourage such actions against us and our directors, officers, employees and agents. While there is no Nevada case law addressing the enforceability of this type of provision, Nevada courts have on prior occasion found persuasive authority in Delaware case law in the absence of Nevada statutory or case law specifically addressing an issue of corporate law. The Court of Chancery of the State of Delaware ruled in June 2013 that choice-of-forum provisions of a type similar to those included in our Amended and Restated Articles of Incorporation are not facially invalid under corporate law and constitute valid and enforceable contractual forum selection clauses. However, if a court were to find the choice-of-forum provision in our Amended and Restated Articles of Incorporation inapplicable to, or unenforceable in respect of, one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could adversely affect our business, financial condition or results of operations.
The elimination of personal liability of our directors and officers under Nevada law and the existence of indemnification rights held by our directors, officers and employees may result in substantial expenses.
Our Amended and Restated Articles of Incorporation eliminate to the furthest extent permitted under Nevada law the personal liability of our directors and officers to us, our stockholders and creditors for damages as a result of any act or failure to act in his or her capacity as a director or officer. Further, our Amended and Restated Articles of Incorporation, our Amended and Restated Bylaws and individual indemnification agreements that we have entered with each of our directors and officers provide that we are obligated to indemnify, subject to certain exceptions, each of our directors or officers to the fullest extent authorized by Nevada law and, subject to certain conditions, to advance the expenses incurred by any director or officer in defending any action, suit or proceeding prior to its final disposition. Those indemnification obligations could expose us to substantial expenditures to cover the cost of settlement or damage awards against our directors or officers, which we may be unable to afford. Further, those provisions and resulting costs may discourage us or our stockholders from bringing a lawsuit against any of our current or former directors or officers for such damages, even if such actions might otherwise benefit our stockholders.
We do not intend to pay cash dividends on our capital stock in the foreseeable future.
We have never declared or paid any cash dividends on our common stock and do not anticipate paying any dividends in the foreseeable future. We currently intend to retain all future earnings to fund the development and growth of our business. Any future payment of cash dividends in the future will be at the discretion of our Board of Directors and will depend on, among other things, our earnings, financial condition, capital requirements, level of indebtedness, statutory and contractual restrictions applying to the payment of dividends and other considerations that the Board of Directors deems relevant. Our stockholders should not expect that we will ever pay cash or other dividends on our outstanding capital stock. Any return to our stockholders will therefore be limited to the appreciation of their stock.
We can issue and have issued shares of preferred stock, which may adversely affect the rights of holders of our common stock.
Our amended and restated Certificate of Incorporation authorizes us to issue up to 10,000,000 shares of preferred stock with designations, rights, and preferences determined from time-to-time by our Board of Directors. Accordingly, our Board of Directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting or other rights superior to those of holders of our common stock. For example, an issuance of shares of preferred stock could:
| |
• | adversely affect the voting power of the holders of our common stock; |
| |
• | make it more difficult for a third party to gain control of us; |
| |
• | discourage bids for our common stock at a premium; |
| |
• | limit or eliminate any payments that the holders of our common stock could expect to receive upon our liquidation; or |
| |
• | otherwise adversely affect the market price or our common stock. |
We have in the past issued, and we may at any time in the future issue, shares of preferred stock. In connection with our June 2016 private placement, we issued 4,963 shares of our Series A convertible preferred stock to certain affiliates of Biotechnology Value Fund, L.P., or BVF, each share of which is convertible into 1,000 shares of our common stock, subject to certain ownership restrictions. In January 2019, we entered into an exchange agreement with BVF, or the Exchange Agreement, to exchange 5,000,000 shares of our common stock previously held by BVF for 5,000 shares of our Series B convertible preferred stock, each share of which is convertible into 1,000 shares of our common stock, subject to certain ownership restrictions. If the holders of our shares of preferred stock convert their shares into common stock, existing holders of our common stock will experience dilution.
We are incurring and will continue to incur significantly increased costs as a result of operating as a public company, and our management is now required to devote substantial time to new compliance initiatives.
We have only been subject to the reporting requirements of the Exchange Act and the other rules and regulations of the SEC since December 2014. As a public company listed on The Nasdaq Stock Market LLC, we are incurring and will continue to incur significant legal, accounting and other expenses that we did not incur as a private company. This would be particularly true if we ceased to be a “smaller reporting company” or an “emerging growth company.” We are subject to the reporting requirements of the Exchange Act, as well as various requirements imposed by the Sarbanes-Oxley Act, rules subsequently adopted by the SEC and Nasdaq to implement provisions of the Sarbanes-Oxley Act, and the Dodd-Frank Wall Street Reform and Consumer Protection Act. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate. The listing requirements of the Nasdaq Stock Market require that we satisfy certain corporate governance requirements relating to director independence, distributing annual and interim reports, stockholder meetings, approvals and voting, soliciting proxies, conflicts of interest and a codes of conduct.
We expect the rules and regulations applicable to public companies to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage. We also expect that we will need to hire additional accounting, finance and other personnel in connection with our efforts to comply with the requirements of being a public company, and our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors and committees thereof or as executive officers.
We are an emerging growth company and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an "emerging growth company" under the JOBS Act. For as long as we continue to be an emerging growth company, we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies including, but not limited to, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, exemptions from the requirements of holding a nonbinding advisory stockholder vote on executive compensation and any golden parachute payments not previously approved, exemption from the requirement of auditor attestation in the assessment of our internal control over financial reporting and exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board. If we do, the information that we provide stockholders may be different than what is available with respect to other public companies. We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected to take advantage of this extended transition period. Since we will not be required to comply with new or revised accounting standards on the relevant dates on which adoption of
such standards is required for other public companies, our financial statements may not be comparable to the financial statements of companies that comply with the effective dates of those accounting standards.
We will remain an emerging growth company until the earliest of (1) the end of the fiscal year in which the market value of our common stock that is held by non-affiliates exceeds $700 million as of the end of the second fiscal quarter, (2) the end of the fiscal year in which we have total annual gross revenues of $1.07 billion or more during such fiscal year, (3) the date on which we issue more than $1 billion in non-convertible debt in a three-year period or (4) December 31, 2019, the end of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement filed under the Securities Act. Decreased disclosures in our SEC filings due to our status as an “emerging growth company” may make it harder for investors to analyze our results of operations and financial prospects. We expect that we will no longer qualify as an emerging growth company on December 31, 2019 as this is the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act.
Having availed ourselves of scaled disclosure available to smaller reporting companies and emerging growth companies, we cannot be certain if such reduced disclosure will make our common stock less attractive to investors.
Under Rule 12b-2 of the Exchange Act, a "smaller reporting company" is a company that is not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company, and, according to the amended definition effective September 10, 2018, had a public float of less than $250 million as of the last business day of its most recently completed second fiscal quarter or, if such public float is less than $700 million, had annual revenues of less than $100 million during the most recently completed fiscal year. Similar to emerging growth companies, smaller reporting companies are permitted to provide simplified executive compensation disclosure in their filings; they are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal controls over financial reporting; and they have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. We qualify as a smaller reporting company. For as long as we continue to be a smaller reporting company, we expect that we will take advantage of the reduced disclosure obligations available to us as a result of those respective classifications. Decreased disclosure in our SEC filings as a result of our having availed ourselves of scaled disclosure may make it harder for investors to analyze our results of operations and financial prospects.
|
| |
Item 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
We currently lease approximately 19,000 square feet of office and laboratory space in Freising, Germany under four agreements, including three leases for space on three floors of the same building and a letter agreement for additional conference room space within the building. We can terminate two of the lease agreements at the end of any quarter with eight months' notice. The other lease agreement will terminate on January 31, 2020 and the letter agreement will terminate on December 31, 2019. In October 2018, Pieris GmbH entered into a lease initially comprising of approximately 96,400 square feet of mixed laboratory and office space in Hallbergmoos, Germany, where we intend to move our Freising operations. This agreement, or the Lease Agreement, provides for an initial term of 150 months, commencing on the date the lessor first delivers the leased property to Pieris GmbH as agreed under the Lease Agreement, which is expected to occur in the fourth quarter of 2019. Pieris GmbH and the lessor are each entitled to terminate the Lease Agreement for due cause.
We lease 3,950 square feet of office space in Boston, Massachusetts under a sublease, or the Sublease, that houses our executive offices, clinical operations, and other operational functions. The Sublease expires on February 27, 2022 or such earlier date pursuant to the termination provisions of the Sublease. We believe that our facilities are sufficient to meet our needs and will look for suitable additional space as and when needed.
As of the date of this Annual Report on Form 10-K, we are not currently involved in any material legal proceedings. However, from time to time, we could be subject to various legal proceedings and claims that arise in the ordinary course of our business activities. Regardless of the outcome, legal proceedings can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
|
| |
Item 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II
|
| |
Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is listed on The Nasdaq Stock Market LLC under the symbol “PIRS” and on June 30, 2015 our common stock began trading on the Nasdaq Capital Market.
Stockholders
As of March 11, 2019, there were 64 and 3 stockholders of record of our common stock and preferred stock, respectively. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial holders represented by these record holders.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
None.
|
| |
Item 6. | SELECTED FINANCIAL DATA |
Not applicable.
|
| |
Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included in this Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties as described under the heading “Forward-Looking Statements” elsewhere in this Annual Report on Form 10-K. You should review the disclosure under the heading “Risk Factors” in this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a clinical-stage biotechnology company that discovers and develops Anticalin-based drugs to target validated disease pathways in unique and transformative ways. Our clinical pipeline includes an inhaled IL-4Rα antagonist Anticalin protein to treat uncontrolled asthma, an IO bispecific targeting HER2 and 4-1BB. Proprietary to us, Anticalin proteins are a novel class of therapeutics validated in the clinic and through partnerships with leading pharmaceutical companies. Our development programs include:
| |
• | PRS-060, our lead respiratory program partnered with AstraZeneca, is a drug candidate that binds to IL-4Rα, thereby inhibiting IL-4 and IL-13, two cytokines known to be key mediators in the inflammatory cascade that drive the pathogenesis asthma and other inflammatory diseases. |
| |
• | PRS-343, our lead IO program, is a fusion protein, comprising a HER2-targeting antibody genetically linked to 4-1BB-targeting Anticalin proteins. PRS-343 is designed to drive tumor localized T-cell activation through tumor-targeted drug clustering mediated by HER2 expressed on tumor cells. This program is the first bispecific T-cell costimulatory agonist to enter clinical development. |
| |
• | We are also developing additional IO drug candidates that are multispecific Anticalin-based fusion proteins designed to engage immunomodulatory targets and consist of a variety of multifunctional biotherapeutics, including PRS-344, a bispecific Anticalin-antibody fusion protein comprising an anti-PD-L1 antibody genetically fused to Anticalin proteins specific for 4-1BB. PRS-344 is being developed as part of our IO collaboration with Servier. |
| |
• | PRS-080 is an Anticalin protein that binds to hepcidin, a natural regulator of iron in the blood. PRS-080 is designed to target hepcidin for the treatment of FID in anemic patients with CKD, particularly in ESRD patients requiring dialysis. |
Our programs are in varying stages:
| |
• | PRS-060, an inhaled IL-4Rα antagonist for moderate-to-severe asthma, was tested in 48 healthy volunteers . The drug candidate was safe and well-tolerated in this study. We continue enrolling subjects in a MAD phase 1 study of the drug candidate versus placebo in mild asthmatics. The MAD study will evaluate safety and tolerability as well as exhaled nitric oxide, an inflammatory marker of inflamed lung epithelial cells. The data from the PRS-060 phase 1 studies will be presented at a future medical meeting. PRS-060 is the lead candidate in our respiratory collaboration with AstraZeneca. We are sponsoring the phase 1 studies and AstraZeneca is funding the costs. AstraZeneca will conduct and fund the phase 2a study, after which we will have separate options to co-develop and co-commercialize the drug candidate in the United States. |
| |
• | We continue to enroll and treat patients in a phase 1 dose-escalation study of PRS-343, a 4-1BB/HER2 bispecific for HER2-positive solid tumors, and intend to report comprehensive data from the study in 2019. In August 2018, we initiated a study with PRS-343 in combination with atezolizumab and intend to report data from this study in 2019. |
| |
• | For our other IO drug candidates and programs, we are conducting activities relating to candidate identification, optimization, and preclinical evaluation. In 2019, we intend to file an IND for PRS-344. |
| |
• | We completed dosing for the phase 2a study of PRS-080 in anemic, hemodialysis-dependent CKD patients in 2018. This study was designed primarily to obtain initial results on the safety, tolerability, and pharmacological activity of 5 once-weekly doses of PRS-080 and, secondarily, to evaluate the effect of repeated PRS-080 administration on hemoglobin levels in this patient population. We intend to present the full data set from this study in 2019. We also plan to share these data with ASKA, at which point ASKA will decide whether to exercise its option to develop and commercialize PRS-080 in Japan and other Asian territories. Additionally, we plan to share the dataset with others for potential partnerships outside of the ASKA territories. PRS-080 has also been investigated in SAD phase 1a and 1b studies, first in healthy subjects (1a), and then in stage 5 CKD patients requiring hemodialysis (1b). |
Our core Anticalin technology and platform were developed in Germany, and we have collaborations with major multi-national pharmaceutical companies. We entered into the Servier Collaboration Agreement in January 2017 in IO and entered into the ASKA Option Agreement in February 2017 for PRS-080 in Japan and other Asian countries. In May 2017, we entered into an alliance with AstraZeneca to treat respiratory diseases and in February 2018 we entered into the Seattle Genetics Collaboration Agreement in IO.
Since inception, we have devoted nearly all of our efforts and resources to our research and development activities and have incurred significant net losses. For the years ended December 31, 2018 and 2017, we reported net loss of $26.8 million and $17.6 million, respectively. As of December 31, 2018, we had an accumulated deficit of $147.1 million. We expect to continue incurring substantial losses for the next several years as we continue to develop our clinical and preclinical drug candidates and programs. Our operating expenses are comprised of research and development expenses and general and administrative expenses.
We have not generated any revenues from product sales to date, and we do not expect to generate revenues from product sales for the foreseeable future. Our revenues for the fiscal years ended December 31, 2018 and 2017 were from license and collaboration agreements with our partners.
A significant portion of our operations are conducted in countries other than the United States. Since we conduct our business in US dollars, our main exposure, if any, results from changes in the exchange rates between the euro and the US dollar. At each period end, we remeasure assets and liabilities to the functional currency of that entity (for example, US dollar payables recorded by Pieris GmbH). Remeasurement gains and losses are recorded in the statement of operations line item Other income (expense), net. All assets and liabilities denominated in euros are translated into US dollars at the exchange rate on the balance sheet date. Revenues and expenses are translated at the weighted average rate during the period. Equity transactions are
translated using historical exchange rates. All adjustments resulting from translating foreign currency financial statements into US dollars are included in accumulated other comprehensive loss.
Key Financial Terms and Metrics
The following discussion summarizes the key factors our management believes are necessary for an understanding of our consolidated financial statements.
Revenues
We have not generated any revenues from product sales to date, and we do not expect to generate revenues from product sales for the foreseeable future. Our revenues for the last two years have been primarily from the license and collaboration agreements with AstraZeneca, Servier, and Seattle Genetics.
The revenues from AstraZeneca, Servier and Seattle Genetics have been comprised primarily of upfront payments, research and development services, and milestone payments. We recognized revenues from upfront payments under these agreements based on multiple-element arrangement guidance as we have determined that the licenses to which the payments related did not have standalone value. Research service revenue is recognized when the costs are incurred and the services have been performed. For revenues from research, development, and commercial milestone payments, if the milestones are deemed substantive and the milestone payments are nonrefundable, such amounts are recognized entirely upon successful accomplishment of the milestones, assuming all other revenue recognition criteria are met. Milestones that are not considered substantive are accounted for as contingent revenue and will be recognized when achieved to the extent we have no remaining performance obligations under the arrangement. We expect our revenues for the next several years to consist of upfront payments, research funding and milestone payments from strategic collaborations we currently have or may establish in the future.
Research and Development Expenses
The process of researching and developing drugs for human use is lengthy, unpredictable, and subject to many risks. We expect to continue incurring substantial expenses for the next several years as we continue to develop our clinical and preclinical drug candidates and programs. We are unable, with any certainty, to estimate either the costs or the timelines in which those expenses will be incurred. Our current development plans focus on the following activities: Our IO programs, currently comprised of PRS-343, PRS-344, and multiple additional proprietary and partnered programs, and PRS-060. These programs consume a large proportion of our current, as well as projected, resources.
Our research and development costs include costs that are directly attributable to the creation of certain of our Anticalin drug candidates and are comprised of:
| |
• | internal recurring costs, such as personnel-related costs (salaries, employee benefits, equity compensation, and other costs), materials and supplies, facilities and maintenance costs; and |
| |
• | fees paid to external parties who provide us with contract services, such as preclinical testing, manufacturing and related testing, and clinical trial activities. |
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, employee benefits, equity compensation, and other personnel-related costs associated with executive, administrative and other support staff. Other significant general and administrative expenses include the costs associated with professional fees for accounting, auditing, insurance costs, consulting and legal services.
Results of Operations
Comparison of Years Ended December 31, 2018 and December 31, 2017
The following table sets forth our revenues and operating expenses for the fiscal years ended December 31, 2018 and 2017 (in thousands):
|
| | | | | | | | |
| | Year Ended December 31, 2018 | | Year Ended December 31, 2017 |
Revenues | | $ | 29,101 |
| | $ | 25,275 |
|
| | | | |
Research and development expenses | | 41,490 |
| | 22,285 |
|
General and administrative expenses | | 18,442 |
| | 17,584 |
|
Total operating expenses | | 59,932 |
| | 39,869 |
|
| | | | |
Interest Income | | 1,962 |
| | 152 |
|
Other income (expense), net | | 1,803 |
| | (2,102 | ) |
Loss before income taxes | | (27,066 | ) | | (16,544 | ) |
Income tax (benefit) provision | | (312 | ) | | 1,103 |
|
Net loss | | $ | (26,754 | ) | | $ | (17,647 | ) |
Revenues
The following table provides a comparison of revenues for the years ended December 31, 2018 and 2017 (in thousands):
|
| | | | | | | | | | | | |
| | Years ended December 31, | | |
| | 2018 | | 2017 | | Increase/(Decrease) |
License Fees | | $ | 26,677 |
| | $ | 11,285 |
| | $ | 15,392 |
|
Research and development services | | 1,762 |
| | 1,403 |
| | $ | 359 |
|
Milestone payments | | 571 |
| | 12,573 |
| | $ | (12,002 | ) |
Other | | 91 |
| | 14 |
| | $ | 77 |
|
Total Revenue | | $ | 29,101 |
| | $ | 25,275 |
| | $ | 3,826 |
|
| |
• | The $15.4 million increase in revenues from license fees in the 12 months ended December 31, 2018 compared to the 12 months ended December 31, 2017 relates to higher revenue across all of our Strategic Partnerships due to an increase in effort in 2018 along with the period in which each collaboration agreement contributed to revenue (AstraZeneca commenced in May 2017 and Seattle Genetics commenced in February 2018). Increases in activities across our Strategic Partnerships was slightly offset by lower revenues on our collaboration with Roche due to revenue recognized upon termination of the agreement in 2018 compared to higher activity on services performed in 2017. |
| |
• | Revenues from research and development services for the 12 months ended December 31, 2018 increased $0.4 million compared to the 12 months ended December 31, 2017 due to higher revenue from research and development services being provided to Servier in Fiscal Year 2018 compared to lower research and development services being provided to Roche in 2017. |
| |
• | The $12.0 million decrease in milestone revenue resulted from the achievement of one AstraZeneca milestone during the 12 months ended December 31, 2017 compared to one Servier milestone achieved during the 12 months ended December 31, 2018. |
Research and Development Expenses
The following table provides a comparison of the research and development expenses for our drug candidates by therapeutic designation for the years ended December 31, 2018 and 2017 (in thousands):
|
| | | | | | | | | | | | |
| | Years ended December 31, | | |
| | 2018 | | 2017 | | Increase/(Decrease) |
Immuno-oncology | | $ | 13,654 |
| | $ | 5,074 |
| | $ | 8,580 |
|
Respiratory | | 8,632 |
| | 5,114 |
| | $ | 3,518 |
|
Anemia | | 1,664 |
| | 2,521 |
| | $ | (857 | ) |
Salaries and other expenses | | 17,540 |
| | 9,576 |
| | $ | 7,964 |
|
Total | | $ | 41,490 |
| | $ | 22,285 |
| | $ | 19,205 |
|
| |
• | the $8.6 million increase in our immuno-oncology program spending period-over-period is due primarily to an increase in clinical trials costs for PRS-343 and an increase in drug product manufacturing primarily for PRS-344. Additionally, 2018 costs included an increase in pre-clinical and lab supply costs for other proprietary and partnered IO programs. Finally, license fees on partnered IO programs increased in 2018 due to license fees owed to TUM for the Seattle Genetics arrangement signed in 2018 along with additional license fees anticipated due to an amended license agreement; |
| |
• | the $3.5 million increase for our respiratory programs period-over-period is due primarily to increases to our ongoing clinical and CMC costs, primarily for PRS-060 and higher license fee payments to TUM for an anticipated revised license agreement; |
| |
• | the $0.9 million decrease for our anemia program, PRS-080, period-over-period is mainly due to lower clinical costs related to the phase 2a study in the second half of 2018; |
| |
• | the $8.0 million increase in other research and development activities is mainly due to increases in personnel expenses, including payroll, bonus and stock compensation. Additionally, preclinical and general lab supply expenses were higher given the increased activities to support further development of our platform technology and other early stage discovery programs. |
General and Administrative Expenses
General and administrative expenses were $18.4 million for the fiscal year ended December 31, 2018 as compared to $17.6 million for the fiscal year ended December 31, 2017. The period-over-period increase is due to increased headcount in our general and administrative functions to support an expanding business which resulted in higher personnel costs, including payroll, bonus and stock compensation. This was partially offset by lower professional services due to transaction fees incurred in 2017 for our AstraZeneca Agreements.
Non-operating income (expense), net
Our non-operating income was $3.8 million for the year ended December 31, 2018 as compared to a net non-operating expense of $2.0 million for the year ended December 31, 2017. This $5.8 million change is mainly a result of net foreign currency transaction gains in 2018 due to a strengthening US dollar compared to the Euro as compared to net foreign currency transaction losses in 2017 due to the strengthening of the Euro against the US dollar and higher US dollar assets subject to foreign currency re-measurement. In addition, we earned approximately $2.0 million in interest on investments, a $1.8 million increase from 2017, as investing activities commenced in the fourth quarter of 2017.
Income tax benefit (expense)
Income tax benefit was $0.3 million for the year ended December 31, 2018 as compared to $1.1 million income tax expense for the year ended December 31, 2017. The income tax expense in 2017 is related to a statutory provision requirement in our Australian jurisdiction resulting from taxable income from the AstraZeneca Agreement signed in 2017. In 2018, the income tax benefit from continuing operations was primarily the result of an offsetting intraperiod tax benefit due to taxable gains in other comprehensive income.
Liquidity and Capital Resources
Through December 31, 2018, we have funded our operations with $382.1 million of cash that has been obtained from the following main sources: $170.5 million from sales of equity; $190.8 million in total payments received under license and collaboration agreements, including $25.0 million for research and development services costs received from our collaboration partners; $14.2 million from government grants and $6.5 million from loans.
As of December 31, 2018, we had a total of $128.1 million in cash, cash equivalents and investments. We have incurred losses in every period since inception including the years ended December 31, 2018 and 2017, respectively, and have a total accumulated deficit of $147.1 million as of December 31, 2018.
In February 2018, we completed an underwritten public offering of our common stock in which we sold 6,325,000 shares of Common Stock, including the exercise in full by the underwriters of their option to purchase an additional 825,000 shares of Common Stock, to the public at a price of $8.00 per share. The offering was completed under the shelf registration statement that was filed on Form S-3 and declared effective by the SEC on August 3, 2016. Net proceeds of the underwritten public offering, after deducting the underwriting discounts and commissions, were $47.6 million, excluding our offering expenses of approximately $0.4 million.
We have several research and development programs underway in varying stages of development and we expect they will continue to require increasing amounts of cash for development, conducting clinical trials, and testing and manufacturing of product material. We expect cash necessary to fund operations will increase significantly over the next several years as we continue to conduct these activities necessary to pursue governmental regulatory approval of our IO programs, including PRS-343 and PRS-344, PRS-060, and our other product candidates.
The following table provides a summary of operating, investing, and financing cash flows for the years ended December 31, 2018 and 2017 respectively (in thousands):
|
| | | | | | | | |
| | Year Ended December 31, 2018 | | Year Ended December 31, 2017 |
Net cash provided by operating activities | | $ | (1,066 | ) | | $ | 49,754 |
|
Net cash used in investing activities | | (8,875 | ) | | (45,875 | ) |
Net cash provided by financing activities | | 48,511 |
| | 4,090 |
|
Net cash used in operating activities of $1.1 million for the year ended December 31, 2018 is comprised principally of operating expenses of $50.6 million, net of non-cash items, offset by aggregate receipts of $50.9 million from AstraZeneca, Servier, and Seattle Genetics and an increase in net working capital of $0.4 million. Net cash provided by operating activities of $49.8 million for the year ended December 31, 2017 is comprised principally of operating expenses of $39.3 million, net of non-cash items, offset by aggregate receipts of $85.7 million from AstraZeneca, Servier, ASKA, and Roche and an increase in net working capital of $2.5 million.
Net cash used in investing activities for the year ended December 31, 2018 decreased by $37.0 million compared to the prior year due mainly to lower purchases of investments.
Net cash provided by financing activities increased by $44.4 million for the year ended December 31, 2018 due primarily to proceeds from the underwritten public offering in February 2018, partially offset by lower proceeds from the exercise of warrants and stock options.
We expect that our existing cash, cash equivalents, and investments will enable us to fund our operational and capital expenditure requirements for at least 12 months from the issuance date of these financial statements. Any requirements for additional capital will depend on many factors, including the following:
| |
• | the scope, rate of progress, results and cost of our clinical studies, preclinical testing and other related activities; |
| |
• | the cost of manufacturing clinical supplies, and establishing commercial supplies, of our drug candidates and any products that we may develop; |
| |
• | the number and characteristics of drug candidates that we pursue; |
| |
• | the cost, timing and outcomes of regulatory approvals; |
| |
• | the cost and timing of establishing sales, marketing and distribution capabilities; |
| |
• | the terms and timing of any collaborative, licensing and other arrangements that we may establish; |
| |
• | the timing, receipt and amount of sales, profit sharing or royalties, if any, from our potential products; |
| |
• | the cost of preparing, filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| |
• | the extent to which we acquire or invest in businesses, products or technologies, although we currently have no commitments or agreements relating to any of these types of transactions. |
Due to the often-volatile nature of the financial markets, equity and debt financing(s) may be difficult to obtain. In addition, any unfavorable development or delay in the progress of our core clinical-stage programs including PRS-343 and PRS-060 could have a material adverse impact on our ability to raise additional capital.
We may seek to raise any necessary additional capital through a combination of private or public equity offerings, debt financings, collaborations, strategic alliances, licensing arrangements and other marketing and distribution arrangements. To the extent that we raise additional capital through marketing and distribution arrangements or other collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our drug candidates, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we raise additional capital through private or public equity offerings, the ownership interest of our existing stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect our stockholders’ rights. If we raise additional capital through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
Contractual Obligations
Leases
We lease office and laboratory space in Freising, Germany as well as office space in Boston, Massachusetts. In Freising, we lease office and laboratory space under four agreements, including three leases for space on three floors of the same building and a letter agreement for additional conference room space within the building. We can terminate two of the lease agreements at the end of any quarter with eight months' notice. The other lease agreement will terminate on January 31, 2020 and the letter agreement will terminate on December 31, 2019. In August 2015, we entered into the Sublease to lease approximately 3,950 square feet in Boston, Massachusetts. The Sublease expires on February 27, 2022 or such earlier date pursuant to the termination provisions of the Sublease.
On October 24, 2018, Pieris GmbH entered into the Lease Agreement with Hallbergmoos Grundvermögen GmbH pursuant to which Pieris GmbH has agreed to lease office and laboratory space located in Hallbergmoos, Germany. Under the Lease Agreement, 96,383 square feet of the leased property is expected to be delivered by the lessor to Pieris GmbH in the fourth quarter of 2019, 8,674 square feet of the leased property is expected to be delivered by the lessor to Pieris GmbH by May 2020 and 22,284 square feet of the leased property is expected to be delivered by the lessor to Pieris GmbH by October 2024. Pieris GmbH has a first right of refusal to lease an additional 13,440 square feet. Pieris GmbH intends to move its operations currently conducted in Freising, Germany to the new leased property.
The Lease Agreement is contingent on the lessor obtaining appropriate building permits from governmental authorities and either party may rescind the Lease Agreement if the lessor does not obtain such permits within a specified period of time. The Lease Agreement provides for an initial term of 150 months, commencing on the date the lessor first delivers the leased property to Pieris GmbH as agreed under the Lease Agreement. Pieris GmbH also has an option to extend the term of the Lease Agreement for two additional 60-month periods. Pieris GmbH may sublease space within the leased property with lessor’s consent, which may not be unreasonably withheld.
Monthly base rent for the initial 105,057 square feet of the leased property, including parking spaces, will total approximately $0.2 million per month, which amount shall be adjusted starting on the second anniversary of the commencement date by an amount equal to the German consumer price index. In addition to the base rent, Pieris GmbH is also responsible for certain administrative and operational costs in accordance with the terms of the Lease Agreement. Pieris GmbH will maintain a security deposit in the amount of three months' rent. Pieris Pharmaceuticals, Inc. will serve as a guarantor for the Lease Agreement.
Pieris GmbH and the lessor are each entitled to terminate the Lease Agreement for due cause. Specifically, the lessor may terminate for Pieris GmbH’s default on rent payments beyond certain amounts, noncompliance with major obligations under the Lease Agreement, and certain bankruptcy and insolvency events.
We record rent expense on a straight-line basis over the lease term period. For the years ended December 31, 2018 and 2017 respectively, we have recognized rent expense under our Boston and Freising lease agreements in an amount of $0.5 million and $0.5 million for the years ended December 31, 2018 and 2017, respectively.
Our contractual commitments of the non-cancellable portion under all operating leases as of December 31, 2018 are as follows (in thousands):
|
| | | |
| Total |
2019 | $ | 613 |
|
2020 | 2,460 |
|
2021 | 2,464 |
|
2022 | 2,347 |
|
2023 | 2,365 |
|
Thereafter | 25,772 |
|
Total minimum lease payments | $ | 36,021 |
|
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, as defined under applicable SEC rules.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires management to make estimates and judgments that affect reported amounts of assets and liabilities as of the date of the balance sheet and reported amounts of revenues and expenses for the periods presented. Management makes estimates and exercises judgment in revenue recognition, share-based payments and income taxes. Judgments must also be made about the disclosure of contingent liabilities, and these estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from those estimates and under different assumptions or conditions.
While our significant accounting policies are more fully described in the notes to our consolidated financial statements, we have identified the following accounting policies that we believe require application of management’s most subjective judgments, often requiring the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Our actual results could differ from these estimates and such differences could be material.
Revenue Recognition
Multiple-element arrangements
When evaluating multiple-element arrangements, we identify the deliverables included within the agreement and evaluate which deliverables represent separate units of accounting based on whether the delivered element has stand-alone value to the collaborator or if the arrangement includes a general right of return for delivered items.
The consideration received is allocated among the separate units of accounting using the relative selling price method, and the applicable revenue recognition criteria are applied to each of the separate units of accounting. We used best estimate of selling price, or BESP, methodology to estimate the selling price for each deliverable and unit of accounting because we do not have vendor specific objective evidence, or VSOE, or third-party evidence, or TPE, of selling price for these deliverables. To determine the estimated selling price of a deliverable, we consider market conditions as well as entity-specific factors, including those factors contemplated in negotiating the agreements, terms of previous collaborative agreements, similar agreements entered into by third parties, market opportunity, estimated development costs, probability of success, and the time needed to commercialize a product candidate pursuant to the license. In validating our best estimate of selling price, we
evaluate whether changes in the key assumptions used to determine the BESP will have a significant effect on the allocation of arrangement consideration among multiple deliverables.
Multiple element arrangements, such as license and development arrangements, are analyzed to determine whether the deliverables, which often include licenses and performance obligations such as research and development services and steering committee services, can be separated or whether they must be accounted for as a combined unit of accounting in accordance with GAAP. We recognize the arrangement consideration allocated to licenses as revenue upon delivery of the license only if the license has stand-alone value. If the license is considered not to have stand-alone value, the license would then be combined with other undelivered elements into a combined unit of accounting and the license payments and payments for performance obligations would be recognized as revenue when the revenue recognition criteria have been satisfied for the last deliverable within the unit of accounting. In the case of combined units of accounting that include delivered licenses and undelivered services to be provided over time, revenue would be recognized over the estimated period during which services will be provided. For units of accounting that include licenses to be delivered upon satisfactory completion of certain research services, revenue is deferred until the license is delivered and the performance obligation is satisfied.
If we are involved in a steering committee, as part of a multiple element arrangement, we assess whether our involvement constitutes a performance obligation or a right to participate. When steering committee services are determined to be performance obligations, we determine the fair value to be allocated to this deliverable and recognize the revenue over the expected term of the development period of the products. Otherwise, the fair value for participation is combined with other research services or performance obligations and is recognized over the term which we expect to complete our aggregate performance obligations.
We recognize arrangement consideration allocated to each unit of accounting when all of the revenue recognition criteria in ASC 605-25 are satisfied for that particular unit of accounting. For each unit of accounting, we must determine the period over which the performance obligations will be performed and revenue will be recognized. If there is no discernible pattern of performance or objectively measurable performance measures do not exist, then we recognize revenue under the arrangement on a straight-line basis over the period we are expected to complete our performance obligations. Conversely, if the pattern of performance over which the service is provided to the customer can be determined and objectively measurable performance measures exist, then we recognize revenue under the arrangement using the proportional performance method. Revenue recognized is limited to the lesser of the cumulative amount of payments received or the cumulative amount of revenue earned as of the period ending date.
Significant management judgment is required in determining the level of effort required under an arrangement and the period over which the we are expected to complete our performance obligations under an arrangement.
The accounting treatment for options granted to collaborators is dependent upon the nature of the option granted to the collaborative partner. Options are considered substantive if, at the inception of an agreement, we are at risk as to whether the collaborative partner will choose to exercise the option(s) to secure additional goods or services. Factors that are considered in evaluating whether options are substantive include the overall objective of the arrangement, benefit the collaborator might obtain from the agreement without exercising the options, cost to exercise the options relative to the total upfront consideration, and additional financial commitments or economic penalties imposed on the collaborator as a result of exercising the options.
In arrangements where options to obtain additional deliverables are considered substantive, we determine whether the optional licenses are priced at a significant and incremental discount. If the prices include a significant and incremental discount, the option is considered a deliverable in the arrangement. However, if not priced at a discount, the option is not considered a deliverable in the arrangement. When a collaborator exercises an option considered to be at a significant and incremental discount to acquire an additional license, the exercise fee that is attributed to the additional license and any incremental discount allocated at inception are recognized in a manner consistent with the treatment of up-front payments for licenses (i.e., license and research services). In the event an option expires unexercised, any incremental discounts deferred at the inception of the arrangement are recognized into revenue upon expiration. For options that are non-substantive, the additional licenses to which the options pertain are considered deliverables upon inception of the arrangement; we apply the multiple-element revenue recognition criteria to determine accounting treatment.
Payments or reimbursements resulting from our research and development efforts in multi-element arrangements, in which our research and development efforts are considered to be a deliverable, are included in allocable consideration and allocated to the units of accounting. These reimbursements are recognized as the services are performed and are presented on a gross basis, so long as there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collection of the related receivable is reasonably assured. Revenue recognized cannot exceed the amount that has been earned and has been billed or is currently
billable. Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue in the accompanying balance sheets.
Milestone payments
At the inception of each agreement that includes milestone payments, we evaluate whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (a) the consideration is commensurate with either (1) the entity’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone, (b) the consideration relates solely to past performance, and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We evaluate factors such as scientific, regulatory, commercial, and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required to achieve the respective milestone, and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment.
We aggregate milestones into four categories: (i) research milestones, (ii) development milestones, (iii) commercial milestones, and (iv) sales milestones. Research milestones are typically achieved upon reaching certain success criteria as defined in each agreement related to developing an Anticalin protein against the specified target. Development milestones are typically reached when a compound reaches a defined phase of clinical research or passes such phase, or upon gaining regulatory approvals. Commercial milestones are typically achieved when an approved pharmaceutical product reaches the status for commercial sale, including regulatory approval. Sales milestones are certain defined levels of net sales by the licensee, such as when a product first achieves global sales or annual sales of a specified amount.
For revenues from research, development, and commercial milestone payments, if the milestones are deemed substantive and the milestone payments are nonrefundable, such amounts are recognized entirely upon successful accomplishment of the milestones, assuming all other revenue recognition criteria are met. Milestones that are not considered substantive are accounted for as contingent revenue and will be recognized when achieved to the extent we have no remaining performance obligations under the arrangement. Revenues from sales milestone payments are accounted for as royalties and are recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met. Royalty payments are recognized in revenues based on the timing of royalty payments earned in accordance with the agreements, which typically is the period when the relevant sales occur, assuming all other revenue recognition criteria are met.
Contingencies
Accruals are recorded for loss contingencies when it is probable that a liability has been incurred and the amount of the related loss can be reasonably estimated. We evaluate, on a quarterly basis, developments in legal proceedings and other matters that could cause an increase or decrease in the amount of the liability that has been accrued previously. Considering facts known at the time of the assessment, we determine whether potential losses are considered reasonably possible or probable and whether they are estimable. Based upon this assessment, we carry out an evaluation of disclosure requirements and consider possible accruals in the financial statements.
Research and Development Expense
Research and development costs are charged to expense as incurred in performing research and development activities. Nonrefundable advance payments for research and development goods or services to be received in the future are deferred and capitalized. The capitalized amounts are expensed as the related goods are delivered or the services are performed. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, pre-clinical and clinical costs, contract services, consulting, depreciation and amortization expense, and other related costs. Costs associated with acquired technology, in the form of upfront fees or milestone payments, are charged to research and development expense as incurred.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating losses and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted statutory tax rates expected to apply to taxable income in the jurisdictions and years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
Based on the level of historical operating results and projections for the taxable income for the future, we have determined that it is more likely than not that our net deferred tax assets will not be realized. Accordingly, we have recorded a full valuation allowance to reduce our net deferred tax assets.
We recognize, measure, present and disclose in our financial statements any uncertain tax positions that we have taken or expect to take on a tax return. We operate in multiple jurisdictions, both within and outside the United States, and may be subject to audits from various tax authorities. Management’s judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities, liabilities for uncertain tax positions, and any valuation allowance recorded against our net deferred tax assets. We will monitor the extent to which our deferred tax assets may be realized and adjust the valuation allowance accordingly.
Our policy is to classify interest and penalties related to unrecognized tax benefits as income tax expense.
Recently Issued Accounting Pronouncements
We review new accounting standards to determine the expected financial impact, if any, that the adoption of each such standard will have. For the recently issued accounting standards that we believe may have an impact on our consolidated financial statements, see “Note 2—Summary of Significant Accounting Policies” in our consolidated financial statements.
Emerging Growth Company and Smaller Reporting Company Status
The JOBS Act establishes a class of company called an “emerging growth company,” which generally is a company whose initial public offering was completed after December 8, 2011 and had total annual gross revenues of less than $1.07 billion during its most recently completed fiscal year. Additionally, Section 12b-2 of the Exchange Act establishes a class of company called a "smaller reporting company," which, effective September 10, 2018, was amended to include companies with a public float of less than $250 million as of the last business day of its most recently completed second fiscal quarter or, if such public float is less than $700 million, had annual revenues of less than $100 million during the most recently completed fiscal year for which audited financial statements are available. Currently, we qualify as both an emerging growth company and a smaller reporting company.
As an emerging growth company and a smaller reporting company, we are eligible and have taken advantage of certain exemptions from various reporting requirements that are not available to public reporting companies that do not qualify for those classifications, including, but not limited to, the following:
| |
• | Any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and financial statements, commonly known as an “auditor discussion and analysis.” |
| |
• | A requirement to hold a non-binding advisory stockholder vote on executive compensation or any golden parachute payments not previously approved by stockholders. |
| |
• | A requirement to comply with the requirement of auditor attestation of management’s assessment of internal control over financial reporting, which is required for other public reporting companies by Section 404 of the Sarbanes-Oxley Act. |
| |
• | An opportunity for reduced disclosure obligations regarding executive compensation in its periodic and annual reports, including without limitation exemption from the requirement to provide a compensation discussion and analysis describing compensation practices and procedures. |
| |
• | An opportunity for reduced financial statement disclosure in registration statements, which must include two years of audited financial statements rather than the three years of audited financial statements that are required for other public reporting companies. Smaller reporting companies are also eligible to provide such reduced financial statement disclosure in annual reports on Form 10-K. |
Emerging growth companies may elect to take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the benefits of this extended transition period. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
For as long as we continue to be an emerging growth company and/or a smaller reporting company, we expect that we will take advantage of the reduced disclosure obligations available to us as a result of those respective classifications. We expect that we will no longer qualify as an emerging growth company on December 31, 2019 as this is the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement under the Securities Act.
|
| |
Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
|
| |
Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Our Consolidated Financial Statements required by this Item are as set forth in Item 15 beginning on page F-3 of this Annual Report on Form 10-K.
|
| |
Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
|
| |
Item 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining “disclosure controls and procedures” as such term is defined in Rule 13a-15(e) of the Exchange Act, as well as for establishing and maintaining “adequate internal control over financial reporting” as such term is defined in Rule 13a-15(f) under the Exchange Act. Our system of internal controls over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the consolidated financial statements in accordance with generally accepted accounting principles.
Because of the inherent limitations surrounding internal controls over financial reporting, our disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. The design of any disclosure controls and procedures also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
Our management, under the supervision of and with the participation of the Chief Executive Officer and Chief Financial Officer, assessed the effectiveness of our internal control over financial reporting and disclosure controls and procedures as of December 31, 2018. In making this assessment, management used the updated criteria set forth in 2013 by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework.
Based on our assessment under the COSO Internal Control-Integrated Framework, management believes that, as of December 31, 2018, our disclosure controls and procedures and internal control over financial reporting were not effective, as described below.
A material weakness is a deficiency, or a combination of deficiencies, in internal controls over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual or interim consolidated financial statements would not be prevented or detected on a timely basis.
In connection with the preparation of our financial statements for the year ended December 31, 2018, we concluded that we had a material weakness relating to our income tax provision process, including the evaluation of any changes resulting from the
recently enacted Tax Act. The material weakness created a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements may not be prevented or detected on a timely basis. The material weakness did not result in any misstatement or correction in the provision for income taxes prior to the issuance of the 2018 consolidated financial statements included in this Form 10-K.
Management intends to implement a remediation plan to address the control deficiency that led to the material weakness. The remediation plan will include enhancing our tax provision process, including the ongoing impact from the Tax Act. We may also retain additional expert assistance, as needed, in the preparation and review of our tax provision.
Remediation of Material Weakness from 2017
As previously disclosed, in connection with the preparation of our financial statements for the year ended December 31, 2017, we concluded that we had a material weakness relating to the financial statement close process due to a combination of deficiencies. The deficiencies resulted from two separate errors that were not identified by management, one related to the classification of certain operating expenses and one related to the reporting of foreign currency re-measurements on investments. To remediate the material weaknesses identified above, we performed the following actions during 2018:
| |
• | Developed and documented formal policies regarding 1) the appropriate accounting for operations and transactions denominated in a foreign currency other than the U.S. Dollar denominated reporting currency 2) the accounting for available-for-sale investments, and 3) the appropriate classification of income statement expenses and other transactions; |
| |
• | Provided training to finance employees upon implementation of these policies; |
| |
• | Hired additional qualified resources within the finance organization to aid in the preparation, supervision, and review during the financial statement close process; |
| |
• | Enhanced and streamlined our financial system, including our chart-of-account structure and financial reporting, to better aid in the timely and accurate review and reporting of financial results. |
As the implementation of the enhanced policies, procedures and controls have functioned effectively for multiple quarters, we concluded that we have remediated the aggregated material weakness previously disclosed from 2017.
Notwithstanding the new material weakness identified as of December 31, 2018, we have concluded that the financial statements and other financial information included in this Annual Report on Form 10-K, fairly present in all material respects our financial condition, results of operations and cash flows as of, and for, the periods presented.
Changes in Internal Control over Financial Reporting
Except for material weakness and remediation activities described above, there have been no changes in internal control over financial reporting identified in connection with the evaluation of such internal control required by Rules 13a-15(d) and 15d-15(d) under the Exchange Act that occurred during the year ended December 31, 2018 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
|
| |
Item 9B. | OTHER INFORMATION |
Not applicable.
PART III
|
| |
Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
MANAGEMENT
Directors and Executive Officers
The table below sets forth information about our directors and executive officers:
|
| | | | | |
Name | | Age | | Position |
Stephen S. Yoder | | 43 |
| | Chief Executive Officer, President and Director |
Allan Reine | | 44 |
| | Senior Vice President, Chief Financial Officer and Treasurer |
Louis A. Matis, M.D. | | 68 |
| | Senior Vice President and Chief Development Officer |
James Geraghty (2)(3) | | 64 |
| | Chairman of the Board of Directors |
Ann Barbier, M.D., Ph.D. (3)(4) | | 54 |
| | Director |
Jean-Pierre Bizzari, M.D. (3)(4) | | 64 |
| | Director |
Peter Kiener, D.Phil. (2)(4) | | 66 |
| | Director |
Christopher Kiritsy (1)(2) | | 54 |
| | Director |
Steven Prelack (2) | | 61 |
| | Director |
Michael Richman (1)(3) | | 58 |
| | Director |
Matthew L. Sherman, M.D. (1)(4) | | 63 |
| | Director |
| |
(1) | Member of the compensation committee |
| |
(2) | Member of the audit committee |
| |
(3) | Member of the nominating and corporate governance committee |
| |
(4) | Member of the science and technology committee |
Business Experience
The following is a brief account of the education and business experience of our current executive officers and directors.
Executive Officers
Stephen S. Yoder. Stephen S. Yoder joined Pieris GmbH as Chief Executive Officer in January 2010 and was appointed to the Board of Directors of Pieris and became Chief Executive Officer and President in December 2014. Prior to joining Pieris GmbH, from July 2003 to December 2010 he led the intellectual property and legal departments at MorphoSys AG, a biotechnology company involved in the development and research of antibodies, as General Counsel. Prior to MorphoSys AG, from September 1999 to June 2003 he worked in several Washington, D.C. law firms, specializing in a life sciences intellectual property practice. Mr. Yoder holds degrees in molecular biology and Spanish from Grove City College and a Juris Doctorate, with honors, from The George Washington University Law School. As an attorney, he is licensed to practice before the USPTO and in the jurisdictions of Maryland and Washington, D.C. We believe that Mr. Yoder adds value to our Board of Directors based on his intimate knowledge of our business plans and strategies of our business and his years of experience in the biotechnology and life sciences industry.
Allan Reine, M.D. Dr. Reine joined Pieris Pharmaceuticals, Inc. as Senior Vice President, Chief Financial Officer and Treasurer in August 2017. Prior to joining Pieris, from August 2012 through August 2017, Dr. Reine was a portfolio manager at Lombard Odier Asset Management, where he ran a healthcare portfolio focused on biotechnology and pharmaceutical companies. Before joining Lombard Odier, Dr. Reine served as a healthcare portfolio manager at various funds, from 2003 through August 2012 including Citi Principal Strategies, SAC Capital, Trivium Capital and Alexandra Investment Management. Dr. Reine began his career in 2001 at CIBC World Markets where he worked in both biotechnology investment banking and biotechnology equity research. Dr. Reine received his M.D. from the University of Toronto, and his Bachelor of Science in Statistical Sciences from the University of Western Ontario.
Louis A. Matis, M.D. Louis A. Matis was appointed Senior Vice President and Chief Development Officer in August 2015. Prior to joining Pieris, Dr. Matis served since June 2011 as Executive Director, Strategic Evaluation at Alexion Pharmaceuticals, where he also served from 1993 to 2000, during which time he advanced to the position of Chief Scientific
Officer and had a leading role in discovering the first-in-class complement inhibitor monoclonal antibody eculizumab. Before re-joining Alexion in 2011, Dr. Matis served as Chief Executive Officer of CGI Pharmaceuticals, Inc. from 2000 to 2006, and of the Immune Tolerance Institute from 2007 to 2010. From 1977 until joining Alexion in 1993, Dr. Matis held senior research and clinical positions at the National Cancer Institute, or the NCI, National Institutes of Health and the FDA Center for Biologics Evaluation and Research. Dr. Matis received a B.A. from Amherst College, an M.D. from the University of Pennsylvania, Perelman School of Medicine, and his clinical training in Internal Medicine at the University of Chicago Hospitals and Clinics and in Medical Oncology at the NCI. Dr. Matis is the author of over 120 publications in major scientific and medical journals and is a co-inventor on multiple patents.
Directors
James Geraghty. James Geraghty joined the Board of Directors of Pieris in May 2017 and was appointed as Pieris's Chairman of the Board of Directors in December 2017. Mr. Geraghty is an industry leader with 30 years of strategic and leadership experience, including more than 20 years as a senior member of executive teams at biotechnology companies developing and commercializing innovative therapies. He was most recently, from May 2013 to October 2016, an Entrepreneur in Residence at Third Rock Ventures, a leading biotech venture and company-formation fund, and previously served as Senior Vice President, North America Strategy and Business Development at Sanofi, which he joined upon its acquisition of Genzyme. Mr. Geraghty spent 20 years at Genzyme, where his roles included Senior Vice President International Development, President of Genzyme Europe and General Manager of Genzyme's cardiovascular business. He is Chairman of the Board of Idera Pharmaceuticals and of Orchard Therapeutics and serves as a Board member of Voyager Therapeutics and of Fulcrum Therapeutics. He is also a member of the BIO Ventures for Global Health Board of Directors. Mr. Geraghty previously served as Chairman of the Board of Juniper Pharmaceuticals. He started his career in healthcare strategy consulting at Bain and Company. A graduate of the Yale Law School, Mr. Geraghty holds an M.S. from the University of Pennsylvania and a B.A. from Georgetown University. We believe that Mr. Geraghty adds value to our Board of Directors due to his strong life sciences pedigree and extensive background in company building.
Ann Barbier, M.D., Ph.D. Dr. Barbier joined the Board of Directors of Pieris in April 2018. She is currently the Chief Medical Officer of Translate Bio. Prior to joining Translate Bio, from June 2015 to October 2017, Dr. Barbier was Vice President of Clinical Development, Rare Genetic Diseases, at Agios Pharmaceuticals, where she led the development program of a small molecule in rare benign hematological diseases. Previously, Dr. Barbier spent seven years at Shire, most recently as Global Clinical Development Lead and Senior Medical Director, where she worked on a variety of rare genetic diseases including lysosomal storage diseases and hereditary angioedema. Her prior experience includes positions at Envivo, Johnson & Johnson and Aventis. During her career, Dr. Barbier has made significant contributions to several approved products such as idursulfase (Hunter syndrome), teriflunomide (multiple sclerosis) and icatibant (hereditary angioedema) and has led several investigational new drug applications for new chemical entities. Additionally, she has authored more than 50 peer-reviewed scientific articles, book chapters and invited reviews. Dr. Barbier received her M.D. and Ph.D. in pharmacology from the University of Gent, Belgium, and a Master of Science from the Free University of Brussels, Belgium. She pursued a postdoctoral fellowship at the University of Tennessee in Memphis. We believe that Dr. Barbier adds value to our Board of Directors due to her significant experience of bringing drug candidates across a wide range of indications through the clinic and to regulatory approval.
Jean-Pierre Bizzari, M.D. Dr. Bizzari joined the Board of Directors of Pieris in May 2015. Dr. Bizzari served as Executive Vice-President, Group Head, Clinical Oncology Development at Celgene Corporation, a role he held from October 2008 until his retirement in December 2015. In this position, Dr. Bizzari was responsible for Celgene’s clinical development and operations-statistics teams across the United States, Europe and Asia/Japan. Dr. Bizzari oversaw the development and approval of a number of leading oncology products including lenalidomide, azacitidine, romidepsin and nab-paclitaxel. In addition, he was Chairman of Celgene’s hematology oncology development committee and a member of the company’s management committee. Prior to his role at Celgene, from 2004 to 2008, Dr. Bizzari was the Vice President, Clinical Oncology Development for Sanofi-Aventis where he oversaw the approval of oxaliplatin, docetaxel and rasburicase. From 2002 to 2004, he was Vice President, Clinical Oncology Development for Sanofi-Synthelabo and from 1993 to 2002 served in the same role for Rhône-Poulenc Rorer, or Aventis. Dr. Bizzari is a member of the Scientific Advisory Board of France’s National Cancer Institute and of Netris Pharma. He is a Board member and Chair of the New Drug Advisory Committee of the European Organisation for Research and Treatment of Cancer. He is also currently a Board member of Halozyme Therapeutics, Inc., Transgene SA, Onxeo SA, Nordic Nanovector ASA, Oxford Bio Therapeutics and Compugen. Dr. Bizzari previously served as a Board member of Celator Pharmaceuticals, Inc. from March 2015 until its acquisition by Jazz Pharmaceuticals plc in July 2016 and of iTeos Therapeutics SA. Dr. Bizzari received his medical degree from the University of Nice (France) and is an oncologist, having trained at La Pitié-Salpêtrière Hospital in Paris, followed by training at the Ontario Cancer Institute and McGill Cancer Center. We believe that Dr. Bizzari adds value to our Board of Directors based on his considerable experience in the pharmaceutical industry and his insight on clinical, regulatory and commercial aspects of drug development, particularly in oncology and global drug approval strategy.
Peter Kiener, D.Phil. Dr. Kiener joined the Board of Directors of Pieris in September 2018. Most recently, Dr. Kiener served as Chief Scientific Officer at Sucampo until the company's acquisition by Mallinckrodt in February 2018. Prior to Sucampo, from August 2013 to September 2014, he served as Chief Scientific Officer of Ambrx Inc., a company focused on developing antibody-drug conjugates. Previously, Dr. Kiener was President and Co-founder of Zyngenia, Inc., a monoclonal antibody company developing drugs for oncology and inflammatory diseases. His prior experience also includes positions at MedImmune LLC, where he was Head of Global R&D, and at Bristol-Myers Squibb. Dr. Kiener currently serves as a Board member of Cue Biopharma, GT Biopharma, Inc. and TetraGenetics, Inc. and is the Chairman of the Board of Managers of Resolve Therapeutics. Dr. Kiener has led or contributed to the development of over 30 different clinical-stage therapeutics and seven approved drugs, including two immunoglobulin-based fusion proteins, one monoclonal antibody, two vaccines, and one bispecific T-cell engager. Additionally, he has published more than 120 papers in peer-reviewed journals and is listed as an inventor on over 60 patents and patent applications. Dr. Kiener received a B.A. in Chemistry from Lancaster University and a D.Phil. in Biochemistry from Sir William Dunn School of Pathology at Oxford University, where he also pursued a postdoctoral fellowship. We believe that Dr. Kiener adds value to our Board of Directors based upon his extensive experience across the entire value chain of biopharmaceutical research and development.
Christopher Kiritsy. Christopher Kiritsy joined the Board of Directors of Pieris in September 2016. Mr. Kiritsy is founder and managing member of Precision Kapital, LLC, a private investment and advisory firm. Prior to forming Precision Kapital, Mr. Kiritsy co-founded Arisaph Pharmaceuticals, Inc., or Arisaph, and served as Arisaph’s President and Chief Executive Officer from 2005 through March 2018. Prior to Arisaph, Mr. Kiritsy served as Executive Vice President, Corporate Development and Chief Financial Officer of Kos Pharmaceuticals, Inc., where he played a key operating role in building the company from start-up to highly profitable, publicly traded, commercial company. During his 10-year tenure at Kos, Mr. Kiritsy spearheaded more than 10 major corporate development transactions and raised approximately $500 million in public equity, including Kos’s initial public offering. Kos was acquired by Abbott Laboratories for $3.7 billion in 2016. Mr. Kiritsy previously served as a Board member and Audit Committee Chair of Melinta Pharmaceuticals, Inc. and he served as a Board member of Arisaph and Chairman of the Board of Avaxia Biologics, Inc. Mr. Kiritsy is a seasoned entrepreneur, who possesses more than 20 years of business and technical experience, previously holding senior management positions in R&D, business development and finance. We believe that Mr. Kiritsy adds value to our Board of Directors based on his considerable experience in the pharmaceutical industry and his expertise in corporate development.
Steven Prelack. Mr. Prelack joined the Board of Directors of Pieris in December 2014. Mr. Prelack is the Senior Vice President and Chief Operating Officer of VetCor, which owns and operates veterinary hospitals across the United States, and has served in this position since June 2012. Prior to that time and since May 2010, Mr. Prelack served at VetCor as Senior Vice President of Operations and as Chief Financial Officer. From 2001 until May 2010, he was the Senior Vice President, Chief Financial Officer and Treasurer of VelQuest Corporation, a provider of automated compliance software solutions for the pharmaceutical industry. He is currently a Board member and Audit Committee Chair of Galectin Therapeutics, Inc., a publicly traded clinical-stage biotechnology company engaged in drug research and development to create new therapies for fibrotic disease and cancer. Mr. Prelack is also currently a Board member and Audit Committee Chair of Aerpio Pharmaceuticals, Inc. a publicly traded clinical-stage biotechnology company engaged in drug research and development to create new therapies for ocular disease. Mr. Prelack also previously served as a Board member and Audit Committee Chair of BioVex Group, Inc., a clinical-stage biotechnology company focused on the development and future commercialization of targeted treatments for cancer and the prevention of infectious disease, which was sold to Amgen in March 2011. Mr. Prelack is a Certified Public Accountant, received a B.B.A. degree from the University of Massachusetts at Amherst in 1979 and is a member of the National Association of Corporate Directors. We believe that Mr. Prelack adds value to our Board of Directors due to his extensive executive leadership experience, director experience within the biotechnology sector and his many years serving in senior financial and operational management roles.
Michael Richman. Mr. Richman joined the Board of Directors of Pieris in December 2014 and has served on the supervisory board of Pieris GmbH since October 2014. He is currently the President and Chief Executive Officer of NextCure, Inc. From June 2008 to July 2015, Mr. Richman was President and Chief Executive Officer of Amplimmune, Inc., a privately held biologics company focused on cancer and autoimmune diseases which was acquired by AstraZeneca in October 2013. From May 2007 to June 2008, he served as President and Chief Operating Officer of Amplimmune, Inc. Prior to such time, Mr. Richman has gained years of experience working in research, intellectual property and business development capacities in companies such as Chiron Corporation (now Novartis), MedImmune, Inc. (now AstraZeneca) and MacroGenics. He is a Board member of Opexa Therapeutics, Inc., a public company, and Madison Vaccines, Inc., a private company. Mr. Richman previously served as a Board member of GenVec, Inc. and Cougar Biotechnology until its acquisition by Johnson & Johnson. Mr. Richman obtained his B.S. in genetics/molecular biology at the University of California at Davis and his M.S.B.A. in international business at San Francisco State University. We believe that Mr. Richman adds value to our Board of Directors due
to his extensive experience in mergers and acquisitions, business development and strategic planning for life science companies, as well as executive leadership and management experience.
Matthew L. Sherman, M.D. Dr. Sherman joined the Board of Directors of Pieris in October 2018. From 2006 through July 2018, Dr. Sherman was Executive Vice President (2015 to 2018) and Chief Medical Officer (2006 to 2018) at Acceleron Pharma. At Acceleron, Dr. Sherman provided executive leadership for medical research, clinical operations, biostatistics, data management, clinical pharmacology and pharmacovigilance. Before joining Acceleron in 2006, Dr. Sherman was Senior Vice President and Chief Medical Officer at Synta Pharmaceuticals, where he oversaw all therapeutic areas, including oncology, inflammatory diseases and immunology. Previously, Dr. Sherman spent over a decade at Wyeth-Ayerst Research/Genetics Institute in numerous clinical research and development roles. Prior to his career in the pharmaceutical and biotechnology industry, Dr. Sherman spent nine years at the Dana-Farber Cancer Institute, ultimately as an Assistant Professor of Medicine. Dr. Sherman currently serves as a Board member of Pulmatrix, Inc. and NewLink Genetics Corp. He has authored more than 255 original articles, review chapters, and abstracts, and is listed as an inventor on 11 issued patents. Dr. Sherman received a B.S. in Chemistry from Massachusetts Institute of Technology and an M.D. from Dartmouth Medical School. He completed his internal medicine residency at Georgetown University Medical Center. We believe that Dr. Sherman adds value to our Board of Directors as he is a physician-scientist with extensive clinical development expertise in oncology, hematology and pulmonary diseases across large pharma, biopharma and venture-funded biotechnology startup companies.
Term of Office of Directors
We currently have authorized nine directors. In accordance with our Amended and Restated Articles of Incorporation and Amended and Restated Bylaws, our Board of Directors is divided into three classes with staggered three-year terms. At each annual meeting of stockholders, the successors to the directors whose terms then expire will be elected to serve until the third annual meeting following the election. Our directors are divided among the three classes as follows:
| |
• | the Class I directors are Jean-Pierre Bizzari, M.D., Christopher Kiritsy and Peter Kiener, D.Phil and their terms will expire at the annual meeting of stockholders to be held in 2021; |
| |
• | the Class II directors are and Steven Prelack, Ann Barbier, M.D., Ph.D. and James Geraghty, and their terms will expire at the annual meeting of stockholders to be held in 2019; and |
| |
• | the Class III directors are Stephen S. Yoder, Michael Richman and Matthew L. Sherman, M.D. and their terms will expire at the annual meeting of stockholders to be held in 2020. |
Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that each class will consist of approximately one-third of the directors.
Family Relationships
There are no family relationships among any of our current or former directors or executive officers.
Involvement in Certain Legal Proceedings
None of our directors, executive officers, significant employees, promoters or control persons has been involved in any legal proceeding in the past 10 years that would require disclosure under Item 401(f) of Regulation S-K promulgated under the Securities Act.
Nominations to the Board of Directors
Director candidates are considered based upon various criteria, including without limitation their broad-based business and professional skills and experiences, expertise in or knowledge of the life sciences industry and ability to add perspectives relating to that industry, concern for the long-term interests of our stockholders, diversity, and personal integrity, judgment, and the need of the Board of Directors. Our Board of Directors has a critical role in guiding our strategic direction and overseeing the strategy of our business, and accordingly, we seek to attract and retain highly qualified directors who have sufficient time to engage in the activities of our Board of Directors and to understand and enhance their knowledge of our industry and business plans.
Our Board of Directors does not have a formal policy with respect to diversity, but an objective of our Board of Directors is to bring to our company a variety of perspectives and skills derived from high quality business and professional experience. Our Board of Directors recognizes its responsibility to ensure that nominees for our Board of Directors possess appropriate qualifications and reflect a reasonable diversity of personal and professional experience, skills, backgrounds and perspectives. We believe that the backgrounds and qualifications of our directors, considered as a group, should provide a composite mix of experience, knowledge and abilities that will allow our Board of Directors to promote our strategic objectives and to fulfill its responsibilities to our stockholders.
The director biographies above indicate each director’s experience, qualifications, attributes and skills that led the Board of Directors to conclude that each director should continue to serve as a member of our Board of Directors. Our Board of Directors believes that each director has had substantial achievement in his professional and personal pursuits and possesses the background, talents and experience that our Board of Directors desires and that will contribute to the best interests of our company and to long-term stockholder value.
Stockholder recommendations for director candidates must be submitted to our corporate secretary at Pieris Pharmaceuticals, Inc., 255 State Street, 9th Floor, Boston, Massachusetts 02109, who will forward all recommendations to the nominating and corporate governance committee. Recommendations for director candidates must be submitted in a timely manner as set forth in our by-laws and must include the information regarding the stockholder and the proposed director candidate and follow all other procedures regarding stockholder nominations of proposed director candidates set forth in the by-laws. The committee will review and evaluate the qualifications of any such proposed director candidate and conduct inquiries it deems appropriate. All proposed director candidates will be evaluated in the same manner, without regard to the source of the initial recommendation.
Committees of the Board of Directors
Our Board has established four standing committees—audit, compensation, nominating and corporate governance and science and technology—each of which operates under a charter that has been approved by our Board. Our Board has determined that all of the members of each of the Board's four standing committees are independent as defined under the rules of the Nasdaq Capital Market. In addition, all members of the audit committee meet the independence requirements contemplated by Rule 10A-3 under the Exchange Act and all members of the compensation committee meet the independence requirements contemplated by Rule 10C-1 under the Exchange Act.
Audit Committee
The audit committee’s main function is to oversee our accounting and financial reporting processes and the audits of our financial statements. This committee’s responsibilities include, among other things:
| |
• | appointing our independent registered public accounting firm; |
| |
• | evaluating the qualifications, independence and performance of our independent registered public accounting firm; |
| |
• | approving the audit and non-audit services to be performed by our independent registered public accounting firm; |
| |
• | reviewing the design, implementation, adequacy and effectiveness of our internal accounting controls and our critical accounting policies; |
| |
• | discussing with management and the independent registered public accounting firm the results of our annual audit and the review of our quarterly unaudited financial statements; |
| |
• | reviewing, overseeing and monitoring the integrity of our financial statements and our compliance with legal and regulatory requirements as they relate to financial statements or accounting matters; |
| |
• | reviewing on a periodic basis, or as appropriate, any investment policy and recommending to our Board any changes to such investment policy; |
| |
• | preparing the report that the SEC requires in our annual proxy statement; |
| |
• | reviewing and approving any related party transactions and reviewing and monitoring compliance with our code of conduct and ethics; and |
| |
• | reviewing and evaluating, at least annually, the performance of the audit committee and its members including compliance of the audit committee with its charter. |
The members of our audit committee are Steven Prelack, James Geraghty, Peter Kiener, D.Phil. and Christopher Kiritsy. Mr. Prelack serves as the chairperson of the committee. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and the Nasdaq Capital Market. Our Board of Directors has determined that Mr. Prelack is an “audit committee financial expert” as defined by applicable SEC rules and has the requisite financial sophistication as defined under the applicable Nasdaq rules and regulations.
Compensation Committee
Our compensation committee reviews and approves policies relating to compensation of our officers and directors and oversees our overall compensation structure, policies and programs. The compensation committee reviews and approves corporate goals and objectives relevant to the compensation of our Chief Executive Officer and other executive officers, evaluates the performance of these officers in light of those goals and objectives and approves the compensation of these officers based on such evaluations. The compensation committee also reviews and approves the issuance of stock options and other awards under our equity plan. The compensation committee will review and evaluate, at least annually, the performance of the compensation committee and its members, including compliance by the compensation committee with its charter.
The members of our compensation committee are Christopher Kiritsy, Michael Richman and Matthew L. Sherman, M.D. Mr. Kiritsy serves as the chairperson of the committee.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for assisting our Board of Directors in discharging the Board’s responsibilities regarding the identification of qualified candidates to become Board members, the selection of nominees for election as directors at our annual meetings of stockholders (or special meetings of stockholders at which directors are to be elected), and the selection of candidates to fill any vacancies on our Board of Directors and any committees thereof. In addition, the nominating and corporate governance committee is responsible for overseeing our corporate governance policies, reporting and making recommendations to our Board of Directors concerning governance matters and oversight of the evaluation of our Board of Directors.
The members of our nominating and corporate governance committee are James Geraghty, Ann Barbier, M.D., Ph.D., Jean-Pierre Bizzari, D.Phil. and Michael Richman. Mr. Geraghty serves as the chairperson of the committee.
Science and Technology Committee
The science and technology committee is responsible for assisting the Board’s oversight of our research and development activities and to advise the Board with respect to strategic and tactical scientific issues. The science and technology committee reviews our overall scientific and research and development strategy, our research and development programs, and cognate external scientific research, discoveries and commercial developments, as appropriate.
The members of our science and technology committee are Ann Barbier, M.D., Ph.D., Jean-Pierre Bizzari, M.D., Peter Kiener, D.Phil. and Mathew L. Sherman, M.D. Dr. Kiener serves as the chairperson of the committee.
SECTION 16(A) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Exchange Act requires directors, executive officers, and persons owning more than 10% of a Company’s class of equity securities registered under Section 12 of the Exchange Act to file reports on a timely basis on the initiation of their status as a reporting person and any changes with respect to their beneficial ownership of such equity securities with the SEC. Executive officers, directors and greater than 10% stockholders are required by SEC regulations to furnish those companies copies of all Section 16(a) forms they file.
Our records reflect all reports which were required to be filed pursuant to Section 16(a) of the Exchange Act were filed on a timely basis during the year ended December 31, 2018, with the exception of three Form 4 filings, which were filed two days late (one Form 4 for Louis Matis, filed February 26, 2018; one Form 4 for Allan Reine, filed February 26, 2018; one Form 4 for Stephen Yoder, filed February 26, 2018).
CODE OF CONDUCT AND ETHICS
We have adopted a Corporate Code of Conduct and Ethics and Whistler Blower Policy that applies to all of our employees, including our chief principal officer and principal financial and accounting officer. The text of the Corporate Code of Conduct and Ethics and Whistler Blower Policy is posted on our website at www.pieris.com, is filed as an exhibit hereto, and will be made available to stockholders without charge, upon request, in writing to the Corporate Secretary at Pieris Pharmaceuticals, Inc., 255 State Street, 9th Floor, Boston, Massachusetts 02109. Disclosure regarding any amendments to, or waivers from, provisions of the code of conduct and ethics that apply to our directors, principal executive and financial officers will be included in a Current Report on Form 8-K within four business days following the date of the amendment or waiver, unless website posting or the issuance of a press release of such amendments or waivers is then permitted by the rules of The Nasdaq Stock Market.
|
| |
Item 11. | EXECUTIVE COMPENSATION |
The following table summarizes the compensation earned in each of our fiscal years ended December 31, 2018 and 2017 by our named executive officers, which consisted of our principal executive officer and our two next most highly compensated executive officers who earned more than $100,000 during the fiscal year ended December 31, 2018 and were serving as executive officers as of such date. We refer to the executive officers listed below as the Named Executive Officers.
Summary Compensation Table
|
| | | | | | | | | | | | | | | | | | | | | |
Name and Principal Position | Year | | Salary | | Bonus ($) | | Option Awards ($) (1) | | All Other Compensation ($) | | Total |
Stephen S. Yoder | 2018 | | $ | 500,000 |
| | $ | 212,500 |
| | $ | 1,866,366 |
| | $ | 42,142 |
| (2) | $ | 2,621,008 |
|
Chief Executive Officer, President | 2017 | | $ | 450,000 |
| | $ | 225,000 |
| | $ | 564,201 |
| | $ | 59,293 |
| (3) | $ | 1,298,494 |
|
Allan Reine, M.D. | 2018 | | $ | 386,250 |
| | $ | 131,325 |
| | $ | 309,145 |
| | $ | 44,542 |
| (4) | $ | 871,262 |
|
Chief Financial Officer | 2017 | | $ | 148,352 |
| (5) | $ | 150,000 |
| | $ | 1,662,130 |
| | $ | 16,704 |
| (6) | $ | 1,977,186 |
|
Louis Matis, M.D. | 2018 | | $ | 391,875 |
| | $ | 133,238 |
| | $ | 574,267 |
| | $ | 24,789 |
| (7) | $ | 1,124,169 |
|
Chief Development Officer | 2017 | | $ | 375,000 |
| | $ | 150,000 |
| | $ | 188,348 |
| | $ | 35,590 |
| (8) | $ | 748,938 |
|
| |
(1) | These amounts represent the aggregate grant date fair value for the option awards granted during the fiscal years presented, determined in accordance with FASB ASC Topic 718. A discussion of the assumptions used in determining grant date fair value may be found in Note 10 to our Financial Statements, included in this Annual Report on Form 10-K. |
| |
(2) | Represents $11,000 for matching contributions we made under our 401(k) Plan and $31,142 for commuting expenses we reimbursed. |
| |
(3) | Represents $10,800 for matching contributions we made under our 401(k) Plan and $48,493 for commuting expenses we reimbursed. |
| |
(4) | Represents $1,289 for matching contributions we made under our 401(k) Plan and $43,253 for commuting expenses we reimbursed. |
| |
(5) | Dr. Reine began employment with us on August 9, 2017. Salary amounts represent the salary actually paid for the fiscal year. |
| |
(6) | Represents $16,704 for commuting expenses we reimbursed. |
| |
(7) | Represents $24,789 for commuting expenses we reimbursed. |
| |
(8) | Represents $35,590 for commuting expenses we reimbursed. |
Narrative Disclosure to Summary Compensation Table
Stephen S. Yoder, Chief Executive Officer
Stephen S. Yoder serves as our President and Chief Executive Officer pursuant to an employment agreement dated December 17, 2014, which provides for a continuous term and may be terminated by either party at any time, provided that if Mr. Yoder resigns he shall provide us with at least 90 days’ prior written notice. Pursuant to his employment agreement, Mr. Yoder is eligible to receive an annual discretionary bonus of up to 40% of Mr. Yoder’s then-effective annual base salary, which the compensation committee increased to a target of up to 50% effective for 2018, based upon achievement of individual and corporate performance objectives as determined by the Board of Directors or a committee thereof. The compensation committee increased Mr. Yoder’s annual base salary to $450,000 for 2017 and $500,000 for 2018. For 2019, the compensation committee increased Mr. Yoder’s annual base salary to $515,000.
Pursuant to his employment agreement, Mr. Yoder is prohibited during the term of the agreement, subject to certain exceptions, from (i) accepting any other employment or consultancy, (ii) serving on the Board of Directors or similar body of any other entity, unless approved by the Chairman of the Board of Directors, and (iii) acquiring, assuming or participating in, directly or indirectly, any financial position, investment or interest known by Mr. Yoder to be adverse or antagonistic to us, our business or prospects, financial or otherwise, or in any competing business.
Mr. Yoder's employment agreement also contains (i) customary confidentiality obligations which are not limited by the term of the agreement, (ii) certain non-compete provisions extending during the term of the agreement and one year thereafter, and (iii) certain non-solicitation provisions during the term of the agreement and for one year thereafter. Mr. Yoder also agreed to assign certain intellectual property rights to us.
Allan Reine, M.D., Chief Financial Officer
Dr. Allan Reine serves as our Senior Vice President, Chief Financial Officer, and Treasurer pursuant to an employment agreement dated August 9, 2017, which provides for a continuous term and may be terminated by either party at any time, provided that if Dr. Reine resigns, he shall provide us with at least 90 days’ written notice. Pursuant to this agreement, Dr. Reine receives a base salary of $375,000 in 2017, which was increased to $386,250 in 2018, and is eligible to receive an annual discretionary bonus award of up to 40% of his then-current base salary, based upon the achievement of specific individual and/or Company-wide performance goals as determined by the Board of Directors, or a committee thereof, in its sole discretion. The compensation committee increased Dr. Reine's annual base salary to $401,700 for 2019. Dr. Reine is entitled to participate in any employee benefit programs, plans and practices on the same terms as other salaried employees on a basis consistent with the participation of other Named Executive Officers.
Dr. Reine, in connection with his employment, was granted an inducement nonqualified stock option to purchase 450,000 shares of our common stock. Twenty-five percent of the option shall vest on the first anniversary of his employment, with the remaining 75% to vest over the next three years in equal installments on a quarterly basis beginning on the last day of the next calendar quarter after the vesting start date.
Dr. Reine was also granted an incentive stock option to purchase up to 50,000 shares of our common stock. The incentive option was based on Dr. Reine substantially meeting his 2017 personal objectives, which were: (1) oversight of our corporate finance functions, (2) contributions to our strategy and leadership, (3) oversight of investor and public relations, and (4) management of our treasury functions. As Dr. Reine achieved all of the objectives, the full 50,000 shares vest as to 25% of the shares on February 20, 2019 and the remaining 75% of the shares shall vest in equal installments on a quarterly basis on the last day of each calendar quarter thereafter, subject in each case to Dr. Reine's continued employment in good standing.
Pursuant to his employment agreement, Dr. Reine is prohibited during the term of the agreement, subject to certain exceptions, from (i) accepting any other employment or consultancy or (ii) serving on the Board of Directors or similar body of any entity, unless such position is approved by the Chief Executive Officer. The agreement with Dr. Reine also contains (i) customary confidentiality obligations which are not limited by the term of the agreement, (ii) certain non-compete provisions extending during the term of the agreement and one year thereafter, (iii) certain non-solicitation provisions during the term of the agreement and for one year thereafter, and (iv) assignment of certain intellectual property rights to us.
Louis Matis, M.D., Chief Development Officer
Dr. Louis Matis serves as our Senior Vice President and Chief Development Officer pursuant to an employment agreement dated July 20, 2015, which provides for a continuous term and may be terminated by either party at any time, provided that if Dr. Matis resigns, he shall provide us with at least 90 days’ written notice. Pursuant to this agreement, the compensation committee increased Dr. Matis's annual base salary to $375,000 for 2017 and to $391,875 for 2018, and Dr. Matis is eligible to
receive an annual discretionary bonus award of up to 40% of his then-current base salary, based upon the achievement of specific individual and/or Company-wide performance goals as determined by the Board or a committee of the Board in its sole discretion. The compensation committee increased Dr. Matis's annual base salary to $403,631 for 2019.
Dr. Matis is entitled to participate in any employee benefit programs, plans and practices on the same terms as other salaried employees on a basis consistent with the participation of other senior executive officers.
Pursuant to his employment agreement, Dr. Matis is prohibited during the term of the agreement, subject to certain exceptions, from (i) accepting any other employment or consultancy, (ii) serving on the Board of Directors or similar body of any other entity, unless approved by the Chief Executive Officer, and (iii) acquiring, assuming or participating in, directly or indirectly, any financial position, investment or interest known by Dr. Matis to be adverse or antagonistic to us, our business or prospects, financial or otherwise, or in any competing business.
The agreement with Dr. Matis also contains (i) customary confidentiality obligations which are not limited by the term of the agreement, (ii) certain non-compete provisions extending during the term of the agreement and one year thereafter and (iii) certain non-solicitation provisions during the term of the agreement and for one year thereafter. Dr. Matis also agreed to assign certain intellectual property rights to us.
2018 Bonus Payments
Our compensation committee approved discretionary cash bonus payments to (i) Mr. Yoder in the amount of $212,500 which was equal to 85% of his target bonus amount, (ii) Dr. Reine in the amount of $131,325, which was equal to 85% of his target bonus amount, and (iii) Dr. Matis in the amount of $133,238, which was equal to 85% of his target bonus amount.
Potential Payments upon Termination or Change in Control
Stephen S. Yoder, Chief Executive Officer and President
Mr. Yoder’s employment agreement provides that if Mr. Yoder’s employment is terminated (i) by us without cause or (ii) by him for good reason, then we must pay Mr. Yoder (i) a lump-sum payment equal to 12 months of his base salary, (ii) a pro rata portion of the bonus for the year in which the termination occurs, based on year-to-date performance as determined by the Board of Directors, or a committee thereof, in its sole discretion, (iii) an amount equal to his health insurance premium, paid directly or as a reimbursement to Mr. Yoder, for up to a maximum of 12 months and (iv) all unvested equity awards then held by Mr. Yoder will immediately vest in full and become exercisable. The severance and acceleration of any unvested options is expressly conditioned on Mr. Yoder executing and delivering to us a release of claims.
Mr. Yoder's employment agreement also provides that, if within 12 months following a change of control Mr. Yoder’s employment is terminated or Mr. Yoder terminates his employment for good reason, and Mr. Yoder executes and delivers to us a release of claims, then he will receive (i) a lump-sum payment equal to 12 months of his base salary at the time of his termination, (ii) his target bonus amount for the year in which the termination occurs, and (iii) an amount equal to 12 months of his health insurance premium, paid directly or as a reimbursement to Mr. Yoder. In addition, all outstanding unvested equity awards will immediately vest in full and become exercisable following termination and any forfeiture restrictions will immediately lapse.
Cause is defined as the occurrence of any of the following events, as determined by the Board of Directors or a committee designated by the Board of Directors, in its sole discretion: (i) Mr. Yoder’s commission of any felony or any crime involving fraud, dishonesty, or moral turpitude under the laws of Germany, the United States or any state thereof; (ii) Mr. Yoder’s attempted commission of, or participation in, a fraud against us; (iii) Mr. Yoder’s intentional, material violation of any contract or agreement between Mr. Yoder and us or of any statutory duty owed to us; (iv) Mr. Yoder’s unauthorized use or disclosure of our confidential information or trade secrets; or (v) Mr. Yoder’s gross misconduct.
Good reason means Mr. Yoder’s resignation from all positions he then holds with us if (i) (a) there is a material diminution in Mr. Yoder’s duties and responsibilities with us; (b) there is a material reduction of Mr. Yoder’s base salary; provided, however, that a material reduction in Mr. Yoder’s base salary pursuant to a salary reduction program affecting all or substantially all of our employees and that does not adversely affect Mr. Yoder to a greater extent than other similarly situated employees shall not constitute good reason; or (c) Mr. Yoder is required to relocate Mr. Yoder’s primary work location to a facility or location that would increase Mr. Yoder’s one-way commute distance by more than 50 miles from Mr. Yoder’s primary work location as of immediately prior to such change, (ii) Mr. Yoder provides written notice outlining such conditions, acts or omissions to us
within 30 days immediately following such material change or reduction, (iii) such material change or reduction is not remedied by us within 30 days following our receipt of such written notice and (iv) Mr. Yoder’s resignation is effective not later than 30 days after the expiration of such 30 day cure period.
Allan Reine, M.D., Chief Financial Officer
Dr. Reine’s employment agreement provides that if Dr. Reine’s employment is terminated (i) by us without cause or (ii) by him for good reason, then Dr. Reine will be entitled to receive (a) six months of his base salary, (b) a bonus equal to his target bonus amount, pro-rated based on the total number of days elapsed in the calendar year as of the termination date if, as of the date of termination, we and Dr. Reine were “on target” to achieve all applicable performance goals, and (c) continuation of COBRA health insurance premiums at our then-normal rate of contribution for 12 months. In addition, outstanding equity awards held by Dr. Reine shall automatically become vested and, if applicable, exercisable and any forfeiture restrictions shall immediately lapse with respect to 75% of the then unvested equity awards. The severance and acceleration of any unvested options is expressly conditioned on Dr. Reine executing and delivering to us a release of claims.
Dr. Reine's employment agreement also provides that, if within 12 months following a change of control Dr. Reine's employment is terminated or Dr. Reine terminates his employment for good reason, and Dr. Reine executes and delivers to us a release of claims, then he will receive (a) 12 months of his base salary, (b) his target bonus amount for the year in which the termination occurs, and (c) continuation of COBRA health insurance premiums at our then-normal rate of contribution for 12 months. In addition, all then outstanding unvested equity awards held by Dr. Reine shall immediately vest in full and, if applicable, become exercisable and all forfeiture restrictions shall immediately lapse.
Louis Matis, M.D., Chief Development Officer
Dr. Matis's employment agreement provides that if Dr. Matis’s employment is terminated (i) by us without cause or (ii) by him for good reason, then Dr. Matis will be entitled to receive (a) six months base salary (b) an amount equal to his target bonus amount, pro-rated based on the total number of days elapsed in the calendar year as of the termination date if, as of the date of termination, we and Dr. Matis were “on target” to achieve all applicable performance goals and (c) continuation of COBRA health insurance premiums at our then-normal rate of contribution for 12 months. In addition, outstanding equity awards held by Dr. Matis shall automatically become vested and, if applicable, exercisable and any forfeiture restrictions shall immediately lapse with respect to75% of the then unvested equity awards.
If, in connection with a change of control of Pieris, Dr. Matis’s employment is terminated without cause or Dr. Matis terminates his employment for good reason, he will be entitled to receive (a) 12 months of his base salary, (b) the target bonus amount for the year of termination and (b) continuation of COBRA health insurance premiums at our then-normal rate of contribution for 12 months. In the case of such a termination in connection with a change in control, outstanding equity awards held by Dr. Matis shall automatically become vested and if, applicable, exercisable and all forfeiture restrictions shall immediately lapse.
“Good Reason” for Dr. Reine and Dr. Matis shall mean the executive’s resignation from all positions he or she then holds with the Company if (i) (A) there is a material diminution in the executive’s duties and responsibilities with the Company or in job title; (B) there is a material reduction of the executive’s base salary; provided, however, that a material reduction in the executive’s base salary pursuant to a salary reduction program affecting all or substantially all of the employees of the Company and that does not adversely affect the executive to a greater extent than other similarly situated employees shall not constitute good reason; or (C) the executive is required to relocate the executive’s primary work location to a facility or location that would increase the executive’s one-way commute distance by more than fifty (50) miles from the executive’s primary work location as of immediately prior to such change, (ii) the executive provides written notice outlining such conditions, acts or omissions to the Company within 30 days immediately following such material change or reduction, (iii) such material change or reduction is not remedied by the Company within 30 days following the Company’s receipt of such written notice and (iv) the executive’s resignation is effective not later than 30 days after the expiration of such 30 day cure period.
“Change of control” for each of the executives shall be deemed to occur (i) when any “person” (as such term is used in Sections 13(d) and 14(d) of the Exchange Act) becomes the “Beneficial Owner” (as defined in Rule 13d-3 under the Exchange Act), directly or indirectly, of securities of Pieris representing 50% or more of the total voting power represented by Pieris’ then outstanding voting securities (excluding for this purpose any such voting securities held by the Pieris or its affiliates or by any employee benefit plan of Pieris) pursuant to a transaction or a series of related transactions which the Board of Directors does not approve; or (ii) a merger or consolidation of Pieris whether or not approved by the Board of Directors, other than a merger
or consolidation which would result in the voting securities of Pieris outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or the parent of such corporation) more than 50% of the total voting power represented by the voting securities of Pieris or such surviving entity or parent of such corporation, as the case may be, outstanding immediately after such merger or consolidation; or (iii) the sale or disposition by Pieris of all or substantially all of its assets in a transaction requiring stockholder approval.
Outstanding Equity Awards at Fiscal Year-End
The table below summarizes the aggregate stock and option awards held by our named executive officers as of December 31, 2018:
|
| | | | | | | | | | | | | |
Name | Number of securities underlying unexercised options (#) exercisable | | Number of securities underlying unexercised options (#) unexercisable | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) (#) unexercisable | | Option exercise price ($) | | Option expiration date |
Stephen S. Yoder Chief Executive Officer, President
| 1,280,000 |
| (1) | — |
| (1) | | | $ | 2.00 |
| | 12/17/2024 |
| 338,250 |
| (2) | 153,750 |
| (2) | | | $ | 1.52 |
| | 2/12/2026 |
| 190,028 |
| | 244,322 |
| (3) | | | $ | 1.99 |
| | 2/23/2027 |
| | | 325,000 |
| (4) | | | $ | 8.56 |
| | 2/20/2028 |
Allan Reine, M.D. Chief Financial Officer | 140,625 |
| | 309,375 |
| (5) | | | $ | 5.00 |
| | 8/9/2027 |
| — |
| | 50,000 |
| (6) | | | $ | 5.00 |
| | 8/9/2027 |
| | | 53,833 |
| (4) | | | $ | 8.56 |
| | 2/20/2028 |
Louis Matis, M.D. Chief Development Officer | 406,250 |
| | 93,750 |
| (7) | | | $ | 3.36 |
| | 8/17/2025 |
| 63,438 |
| | 81,562 |
| (3) | | | $ | 1.99 |
| | 2/23/2027 |
| | | 100,000 |
| (4) | | | $ | 8.56 |
| | 2/20/2028 |
| |
(1) | The option award has a grant date of December 17, 2014 and vested pursuant to the following schedule: 25% of the option vested immediately upon grant on December 17, 2014 and the remaining 75% of the option vested ratably over three years in equal installments on a quarterly basis thereafter. The option award is now fully vested. |
| |
(2) | The option award has a grant date of February 12, 2016 and vests pursuant to the following schedule: 25% of the option vested on the one-year anniversary of the grant date and the remaining 75% of the option shall vest ratably over three years in equal installments on a quarterly basis thereafter. |
| |
(3) | The option award has a grant date of February 23, 2017 and vests pursuant to the following schedule: 25% of the option vests on the one-year anniversary of the grant date and 75% of the option shall vest ratably over three years in equal installments on a quarterly basis beginning on the last day of the next calendar quarter after the date of grant. |
| |
(4) | The option award has a grant date of February 20, 2018 and vests pursuant to the following schedule: 25% of the option vested on January 1, 2019 and the remaining 75% of the option shall vest 6.25% of the option shares at the end of each successive three-month period thereafter. |
| |
(5) | The option award has a grant date of August 9, 2017 and vests pursuant to the following schedule: 25% of the option vests on the one-year anniversary of the grant date and 75% of the option shall vest ratably over three years in equal installments on a quarterly basis beginning on the last day of the next calendar quarter after the date of grant. |
| |
(6) | The option award has a grant date of August 9, 2017 and a grant of an option for up to 50,000 shares of common stock could be earned based on achievement of 2017 personal objectives. The compensation committee determined on February 20, 2018 that all of the objectives had been met and 100% of the award was earned. Therefore, 25% of the shares vested on February 20, 2019 and the remaining 75% of the shares shall vest in equal installments on a quarterly basis on the last day of the next calendar quarter thereafter. |
| |
(7) | The option award has a grant date of August 17, 2015 and vests pursuant to the following schedule: 25% of the options vested on the one-year anniversary of the grant date and the remaining 75% of the option vested ratably over three years in equal installments on a quarterly basis thereafter. The option award is now fully vested. |
Director Compensation
The table below summarizes all compensation earned by each of our non-employee directors for services performed during our fiscal year ended December 31, 2018. Mr. Yoder is not in the table below because he receives no separate compensation for his services as a director of our company, and all of the compensation earned by Mr. Yoder during our 2018 fiscal year as an executive officer of our company is reflected in the Summary Compensation Table above.
|
| | | | | | | | | | | |
Name | Fees earned or paid in cash ($) | | Option awards ($) (10) | | Total ($) |
James Geraghty (1) | $ | 74,531 |
| | $ | 125,324 |
| | $ | 199,855 |
|
Michael Richman (2) | $ | — |
| | $ | 144,404 |
| | $ | 144,404 |
|
Steven Prelack (3) | $ | 50,000 |
| | $ | 100,259 |
| | $ | 150,259 |
|
Jean-Pierre Bizzari, M.D. (4) | $ | 39,693 |
| | $ | 100,259 |
| | $ | 139,952 |
|
Christopher Kiritsy (5) | $ | 52,500 |
| | $ | 100,259 |
| | $ | 152,759 |
|
Ann Barbier, M.D., Ph.D. (6) | $ | 25,471 |
| | $ | 128,557 |
| | $ | 154,028 |
|
Peter Kiener, D.Phil. (7) | $ | 14,238 |
| | $ | 91,277 |
| | $ | 105,515 |
|
Matthew L. Sherman, M.D. (8) | $ | 8,678 |
| | $ | 91,277 |
| | $ | 99,955 |
|
Julian Adams (9) | $ | 24,022 |
| | $ | 100,259 |
| | $ | 124,281 |
|
| |
(1) | As of December 31, 2018, James Geraghty held option awards for 95,000 shares at exercise prices ranging from $3.94 to $7.72. |
| |
(2) | As of December 31, 2018, Michael Richman held option awards for 212,044 shares at exercise prices ranging from $1.59 to $7.72. |
| |
(3) | As of December 31, 2018, Steven Prelack held option awards for 90,000 shares at an exercise price ranging from $1.59 to $7.72. |
| |
(4) | As of December 31, 2018, Jean-Pierre Bizzari, M.D. held option awards for 90,000 shares at an exercise price ranging from $1.59 to $7.72. |
| |
(5) | As of December 31, 2018, Christopher Kiritsy held option awards for 70,000 shares at exercise prices ranging from $1.59 to $7.72. |
| |
(6) | As of December 31, 2018, Ann Barbier, M.D., Ph.D. held option awards for 30,000 shares at an exercise price of $6.43. |
| |
(7) | As of December 31, 2018, Peter Kiener, D.Phil. held option awards for 30,000 shares at an exercise price of $4.46. |
| |
(8) | As of December 31, 2018, Matthew Sherman held option awards for 30,000 shares at an exercise price of $4.46. |
| |
(9) | As of December 31, 2018, Julian Adams did not hold any option awards. |
| |
(10) | These amounts represent the aggregate grant date fair value of option awards granted to each director in fiscal year 2017 computed in accordance with FASB ASC Topic 718. A discussion of the assumptions used in determining grant date fair value may be found in Note 10 to our Financial Statements, included in this Annual Report on Form 10-K. |
Our director compensation program is administered by our Board with the assistance of the compensation committee. The compensation committee conducts an annual review of director compensation and makes recommendations to the Board with respect thereto. The compensation committee has engaged the services of Pearl Meyer & Partners, or Pearl Meyer, a national executive compensation consulting firm, to review and provide recommendations concerning our non-employee director compensation policy. On January 11, 2015, our Board of Directors approved a director compensation policy applicable to our non-employee directors. The January 2015 policy provided for annual cash compensation of $25,000 for each non-employee member of the Board of Directors. In addition, the policy provides that the chair of the audit committee will receive additional annual cash compensation of $15,000; the chair of the compensation committee will receive additional annual cash compensation of $10,000; and the chair of the nominating and corporate governance committee will receive additional annual cash compensation of $7,500; and each member of the audit committee other than the chair will receive $7,500 additional cash compensation, each member of the compensation committee other than the chair will receive $5,000 additional cash compensation and each member of the nominating and corporate governance committee other than the chair will receive $3,750 additional cash compensation. In March 2017, our Board of Directors approved an amendment to the policy to increase the amount of the annual cash retainer paid to each non-employee director from $25,000 to $35,000 and to provide that the Chairperson of the Board of Directors would receive additional annual cash compensation of $25,000. In November 2017, this policy was amended and restated to increase the additional annual cash compensation to be paid to the Chairperson of the Board to $30,000. In October 2018, this policy was further amended to specify that (i) the chair of the science and technology committee would receive, in addition to annual cash compensation for non-employee directors, annual cash compensation of $10,000, (ii) each member of the science and technology committee other than the chair would receive, in addition to annual cash compensation for non-employee directors, $5,000 annual cash compensation, and (iii) that the annual cash compensation for the chair of the Board of Directors is in addition to the annual retainer amount for a member of the Board of Directors, and (iv) the annual cash compensation amounts paid to chairs of each committee of the Board of Directors, including the science and technology committee, are in lieu of the annual cash compensation payable to a member of the applicable committee of the Board of Directors
Since adoption of the policy in 2015 upon appointment, each new non-employee director receives an option to purchase 30,000 shares of our common stock and since 2016 each ongoing non-employee director receives an option to purchase 20,000 shares on January 25 of each year, up from 15,000 shares in 2015. Under the amended and restated November 2017 policy, the Chairperson of the Board will also receive an additional annual option to purchase 5,000 shares and any newly appointed Chairperson of the Board will receive an initial additional option to purchase 40,000 shares. All options granted under the policy shall have an exercise price equal to the fair market value of our common stock on the grant date and terminate 10 years after the grant date. The October 2018 amended and restated policy changed the vesting period of such awards from vesting in equal quarterly installments at the end of each quarter following the grant date until the end of the fiscal year in which the grant was made, to vesting one year after the initial date of grant. The initial additional option granted to the Chairperson vests as to 25% on the first anniversary of the date of the Chairman's appointment as Chairman, with the remaining 75% vesting in 12 equal quarterly installments at the end of each full fiscal quarter following the initial vesting date. In October 2018, our Board of Directors approved an amendment to the vesting period for non-employee director option awards such that both new and annual director awards vest on the one-year anniversary of each respective grant.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth the number of shares of our common stock beneficially owned as of February 28, 2019, by (i) each of our current directors and named executive officers, (ii) all executive officers and directors as a group, and (iii) each person known by us to be the beneficial owner of more than 5% of the outstanding shares of our common stock. We have determined beneficial ownership in accordance with applicable rules of the SEC, which generally provide that beneficial ownership includes voting or investment power with respect to securities. Except as indicated by the footnotes to the table below, we believe, based on the information furnished to us, that the persons named in the table have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws.
The information set forth in the table below is based on 54,151,219 shares of our common stock issued and outstanding on of December 31, 2018. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed to be outstanding all shares of common stock subject to options, warrants or other convertible securities held by that person that are currently exercisable or will be exercisable within 60 days after February 28, 2019. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person. Except as otherwise noted in the footnotes below, the address for each person listed in the table below, solely for purposes of filings with the SEC, is c/o Pieris Pharmaceuticals, Inc., 225 State Street, 9th Floor, Boston, Massachusetts 02109.
|
| | | | | | |
Name and Address of Beneficial Owner | | Number of Shares Beneficially Owned | | Percentage Beneficially Owned |
5%+ Stockholders: | | | | |
Biotechnology Value Fund, L.P. (1) | | 5,409,707 |
| | 9.99 | % |
Directors and Named Executive Officers: | | | | |
Stephen S. Yoder (2) | | 1,953,425 |
| | 3.61 | % |
Louis Matis (3) | | 545,000 |
| | 1.01 | % |
Allan Reine (4) | | 219,083 |
| | * |
|
James Geraghty (5) | | 87,500 |
| | * |
|
Ann Barbier (6) | | 22,500 |
| | * |
|
Jean-Pierre Bizzari (7) | | 90,000 |
| | * |
|
Peter Kiener | | — |
| | * |
|
Christopher Kiritsy (8) | | 75,000 |
| | * |
|
Steven Prelack (9) | | 90,000 |
| | * |
|
Michael Richman (10) | | 212,044 |
| | * |
|
Matthew Sherman | | — |
| | * |
|
All Current Directors and Executive Officers as a Group (11 persons) (11) | | 3,294,552 |
| | 6.08 | % |
| |
(1) | This information is based on a Form 4 filed with the SEC on or about February 1, 2019 and information available to us which includes (i) 1,891,870 shares of Common Stock, (ii) 2,907,000 shares of common stock issuable upon the conversion of Series A Preferred Stock and (iii) 610,837 shares of common stock issuable upon the conversion of Series B Preferred Stock. The address of the principal business and office of BVF Inc. and certain of its affiliates is 1 Sansome Street, 30th Floor, San Francisco, California, 94104. BVF Inc. and its related entities beneficially hold (i) 1,891,870 shares of common Stock, (ii) 2,907 shares a Series A Convertible Preferred Stock, which is convertible into 2,907,000 shares of common stock, (iii) 5,000 shares a Series B Convertible Preferred Stock, which is convertible into 5,000,000 shares of common stock, and (iii) warrants exercisable for 2,374,200 shares of common stock. The Series A and Series B Preferred Stock may not be converted and the warrants may not be exercised if, after such conversion or exercise, BVF Inc. and its affiliates would beneficially own more than 9.99% of the number of shares of common stock then issued and outstanding. As a result of the limitation in the previous sentence, (i) 4,389,163 shares of common stock issuable upon the conversion of Series B Preferred Stock and (ii) 2,374,200 shares of common stock issuable upon the exercise of warrants are excluded from the table above. BVF Partners L.P., or Partners, is the general partner of Biotechnology Value Fund, L.P., or BVF, and Biotechnology Value Fund II, L.P., or BVF II; Partners is the investment manager of Biotechnology Value Trading Fund OS LP, or Trading Fund OS, and is the sole member of BVF Partners OS Ltd, or Partners OS. BVF Inc. is the general partner of Partners, and Mark N. Lampert is a director and officer of BVF Inc. Partners OS disclaims beneficial ownership of the shares of common stock beneficially owned by Trading Fund OS. Each of Partners, BVF Inc. and Mr. Lampert disclaims beneficial ownership of the shares of common stock beneficially owned by BVF, BVF II, Trading Fund OS, and certain Partners managed accounts. |
| |
(2) | Includes 6,000 shares of our common stock and 1,947,425 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(3) | Includes 10,000 shares of our common stock and 535,000 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(4) | Includes 65,000 shares of our common stock and 154,083 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(5) | Includes 20,000 shares of our common stock and 67,500 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(6) | Includes 22,500 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(7) | Includes 90,000 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(8) | Includes 10,000 shares of our common stock and 65,000 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(9) | Includes 90,000 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(10) | Includes 212,044 shares issuable upon the exercise of options to purchase common stock, which are exercisable within 60 days of February 28, 2019. |
| |
(11) | See notes 3 through 10 above. |
Securities Authorized for Issuance under Equity Compensation Plans
Equity Compensation Plan Information
The following table sets forth information as of December 31, 2018 with respect to compensation plans under which our equity securities are authorized for issuance:
|
| | | | | | | | | | | |
Plan Category | | Number of securities to be issued upon exercise of outstanding options, warrants and rights | | Weighted-average exercise price of outstanding options, warrants and rights | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a) | |
Equity compensation plans approved by security holders | | 6,850,047 |
| (1) | $ | 3.76 |
| | 3,330,034 |
| (2) |
Equity compensation plans not approved by security holders | | 1,125,000 |
| (3) | $ | 4.41 |
| | — |
| |
Total | | 7,975,047 |
| | | | 3,330,034 |
| |
| |
(1) | All shares are to be issued pursuant to our 2018 Employee, Director and Consultant Equity Incentive Plan. |
| |
(2) | Comprised of 2,830,034 shares available for issuance under our 2018 Employee, Director and Consultant Equity Incentive Plan and 500,000 shares available for sale under the 2018 Employee Stock Purchase Plan. |
| |
(3) | Pursuant to a stock option agreement with Dr. Matis, dated August 17, 2015, Dr. Matis was granted an option to purchase 500,000 shares of Common Stock at a price per share of $3.36, as an inducement material to his entering into employment with us. The grant has a term of 10 years and is subject to a vesting schedule of four years, with 25% of the shares vesting on August 17, 2016 and 6.25% of the shares vesting each quarter thereafter, subject to his continued employment with us. |
Pursuant to a stock option agreement with Dr. Reine dated August 9, 2017, Dr. Reine was granted an option to purchase 450,000 shares of Common Stock at a price per share of $5.00, as an inducement material to his entering into employment with us. The grant has a term of 10 years and is subject to a vesting schedule of four years, with 25% of the shares vesting on August 17, 2018 and 6.25% of the shares vesting each quarter thereafter, subject to his continued employment with us.
Pursuant to a stock option agreement with Dr. Bruns, dated October 12, 2017, Dr. Bruns was granted an option to purchase 175,000 shares of Common Stock at a price per share of $5.87, as an inducement material to his entering into employment with us. The grant has a term of 10 years and is subject to a vesting schedule of four years, with 25% of the shares vesting on October 12, 2018 and 6.25% of the shares vesting each calendar quarter beginning March 31, 2019, subject to his continued employment with us.
|
| |
Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Related Party Transactions
Except as described below and in the compensation arrangements described in Part II, Item 11 of this Annual Report on Form 10-K, in the fiscal years ended December 31, 2018 and December 31, 2017, there has not been, nor is there currently proposed,
any transaction to which we are or were a party in which the amount involved exceeds the lesser of $120,000 and 1% of the average of our total assets at year-end for the last two completed fiscal years, and in which any of our current directors, executive officers, holders of more than 5% of any class of our voting securities or any of their respective affiliates or immediate family members, had, or will have, a direct or indirect material interest.
We have entered into indemnification agreements with each of our directors and executive officers. Each of those indemnification agreements is in the form approved by our Board of Directors. Those indemnification agreements require that, under the circumstances and to the extent provided for therein, we indemnify such persons to the fullest extent permitted by applicable law against certain expenses and other amounts incurred by any such person as a result of such person being made a party to certain actions, suits and proceedings by reason of the fact that such person is or was a director, officer, employee or agent of our company, any entity that was a predecessor corporation of our company or any of our affiliates. The rights of each person who is a party to such an indemnification agreement are in addition to any other rights such person may have under applicable Nevada law, our Amended and Restated Articles of Incorporation, our Amended and Restated Bylaws, any other agreement, a vote of our stockholders, a resolution adopted by our Board of Directors or otherwise.
Review, Approval or Ratification of Transactions with Related Persons
Pursuant to the written charter of our audit committee, the audit committee is responsible for reviewing and approving all transactions in which we are a participant and in which any parties related to us, including our executive officers, our directors, beneficial owners of more than 5% of our securities, immediate family members of the foregoing persons and any other persons whom our Board of Directors determines may be considered related parties under Item 404 of Regulation S-K, has or will have a direct or indirect material interest. All of the transactions described in this section occurred prior to the adoption of the audit committee charter.
Director Independence
Our Board of Directors undertook a review of the composition of our Board of Directors and independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our Board of Directors has determined that each of James Geraghty, Ann Barbier, M.D., Ph.D., Jean-Pierre Bizzari, M.D., Peter Kiener, D.Phil., Christopher Kiritsy, Steven Prelack, Michael Richman, and Matthew L. Sherman, M.D. would qualify as “independent” as that term is defined by Nasdaq Listing Rule 5605(a)(2). Stephen S. Yoder would not qualify as “independent” under applicable Nasdaq Listing Rules applicable to the Board of Directors generally or to separately designated Board committees because he currently serves as our Chief Executive Officer. In making such determinations, our Board of Directors considered the relationships that each of our non-employee directors has with our company and all other facts and circumstances deemed relevant in determining independence, including the beneficial ownership of our capital stock by each non-employee director.
Subject to some exceptions, Nasdaq Listing Rule 5605(a)(2) provides that a director will only qualify as an “independent director” if, in the opinion of our Board of Directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director, and that a director cannot be an “independent director” if (a) the director is, or in the past three years has been, an employee of ours; (b) a member of the director’s immediate family is, or in the past three years has been, an executive officer of ours; (c) the director or a member of the director’s immediate family has received more than $120,000 per year in direct compensation from us within the preceding three years, other than for service as a director or benefits under a tax-qualified retirement plan or non-discretionary compensation (or, for a family member, as a non-executive employee); (d) the director or a member of the director’s immediate family is a current partner of our independent public accounting firm, or has worked for such firm in any capacity on our audit at any time during the past three years; (e) the director or a member of the director’s immediate family is, or in the past three years has been, employed as an executive officer of a company where one of our executive officers serves on the compensation committee; or (f) the director or a member of the director’s immediate family is an executive officer, partner or controlling stockholder of a company that makes payments to, or receives payments from, us in an amount which, in any 12-month period during our past three fiscal years, exceeds the greater of 5% of the recipient’s consolidated gross revenues for that year or $200,000 (except for payments arising solely from investments in our securities or payments under non-discretionary charitable contribution matching programs). Additionally, in order to be considered an independent member of an audit committee under Rule 10A-3 under the Exchange Act, a member of an audit committee may not, other than in his or her capacity as a member of the audit committee, the Board of Directors, or any other committee of the Board of Directors, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the applicable company or any of its subsidiaries or otherwise be an affiliated person of the applicable company or any of its subsidiaries. To be considered an independent member of the compensation committee under Rule 10C-1 under the Exchange Act, the Board must consider and determine whether a director has a relationship to such company which is material to that director's ability to be independent from management in
connection with the duties of a compensation committee member, including, but not limited to: the source of compensation of the director, including any consulting advisory or other compensatory fee paid by such company to the director; and whether the director is affiliated with the company or any of its subsidiaries or affiliates.
|
| |
Item 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
On April 4, 2016, the audit committee engaged Ernst & Young LLP, or E&Y LLP, as our independent registered public accounting firm to act as the principal accountant to audit our financial statements.
The following table presents fees for professional audit services rendered by E&Y LLP for the audit of our annual financial statements for the years ended December 31, 2018 and 2017 and fees billed for other services rendered by E&Y LLP during the period:
|
| | | | | | | | |
| | 2018 | | 2017 |
Audit fees: (1) | | $ | 1,172,900 |
| | $ | 995,650 |
|
Audit related fees: | | — |
| | — |
|
Tax fees: | | — |
| | — |
|
All other fees: | | — |
| | — |
|
Total | | $ | 1,172,900 |
| | $ | 995,650 |
|
| |
(1) | Audit fees consisted of audit work performed on the annual financial statements, review of quarterly financial statements, as well as work generally only the independent registered public accounting firm can reasonably be expected to provide, such as the provision of consents in connection with the filing of registration statements, Current Reports on Form 8-K and related amendments and statutory audits. |
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Public Accountant
Consistent with SEC policies regarding auditor independence, the audit committee has responsibility for appointing, setting compensation and overseeing the work of our independent registered public accounting firm. In recognition of this responsibility, the audit committee has established a policy to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm.
Prior to engagement of an independent registered public accounting firm for the next year’s audit, management will submit an aggregate of services expected to be rendered during that year for each of four categories of services to the audit committee for approval.
1. Audit services include audit work performed on the annual financial statements, as well as work that generally only an independent registered public accounting firm can reasonably be expected to provide, including comfort letters, statutory audits, and attest services and consultation regarding financial accounting and/or reporting standards.
2. Audit-Related services are for assurance and related services that are traditionally performed by an independent registered public accounting firm, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements.
3. Tax services include all services performed by an independent registered public accounting firm’s tax personnel except those services specifically related to the audit of the financial statements, and includes fees in the areas of tax compliance, tax planning, and tax advice.
4. Other Fees are those associated with services not captured in the other categories. We generally do not request such services from our independent registered public accounting firm.
Prior to engagement, the audit committee pre-approves these services by category of service. The fees are budgeted and the audit committee requires our independent registered public accounting firm and management to report actual fees versus the budget at year end by category of service. During the year, circumstances may arise when it may become necessary to engage
our independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the audit committee requires pre-approval before engaging our independent registered public accounting firm.
The audit committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the audit committee at its next scheduled meeting.
PART IV
|
| | |
Item 15. | | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
| |
Item 15(a). | | The following documents are filed as part of this Annual Report on Form 10-K: |
| |
Item 15(a)(1) and (2) | | See “Index to Consolidated Financial Statements” on page F-1 to this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto. |
| |
Item 15(a)(3) | | Exhibits |
| |
| | The following is a list of exhibits filed as part of this Annual Report on Form 10-K. |
|
| | | | | | | | | |
Exhibit Number | | Exhibit Description | | | Incorporated by Reference herein from Form or Schedule | | Filing Date | | SEC File / Registration Number |
| | | | | | | | | |
| | Acquisition Agreement, dated as of December 17, 2014, by and among the Registrant, Pieris AG and the former stockholders of Pieris AG named therein | | | Form 8-K (Exhibit 2.1) | | December 18, 2014 | | 333-190728 |
| | | | | | | | | |
| | Amended and Restated Articles of Incorporation of the Registrant | | | Form 8-K (Exhibit 3.1) | | December 18, 2014 | | 333-190728 |
| | | | | | | | | |
| | Certificate of Designation of Series A Convertible Preferred Stock | | | Form 10-Q (Exhibit 3.1) | | August 11, 2016 | | 001-37471 |
| | | | | | | | | |
| | Certificate of Designation of Series B Convertible Preferred Stock
| | | Form 8-K (Exhibit 3.1) | | February 4, 2019 | | 001-37471 |
| | | | | | | | | |
| | Amended and Restated Bylaws of the Registrant | | | Form 8-K (Exhibit 3.2) | | December 18, 2014 | | 333-190728 |
| | | | | | | | | |
| | Form of Common Stock certificate | | | Form 8-K (Exhibit 4.1) | | December 18, 2014 | | 333-190728 |
| | | | | | | | | |
| | Form of Common Stock certificate | | | Form 10-K (Exhibit 4.2) | | March 23, 2016 | | 001-37471 |
| | | | | | | | | |
| | 2014 Employee, Director and Consultant Equity Incentive Plan | # | | Form 8-K (Exhibit 10.1) | | December 18, 2014 | | 333-190728 |
| | | | | | | | | |
| | Form of Stock Option Award Agreement under the Registrant’s 2014 Employee, Director and Consultant Equity Incentive Plan | # | | Form 8-K (Exhibit 10.2) | | December 18, 2014 | | 333-190728 |
| | | | | | | | | |
| | 2016 Employee, Director and Consultant Equity Incentive Plan | # | | Form 8-K (Exhibit 10.1) | | July 1, 2016 | | 001-37471 |
| | | | | | | | | |
| | Form of Stock Option Award Agreement under the Registrant’s 2016 Employee, Director and Consultant Equity Incentive Plan | # | | Form 10-K (Exhibit 10.4) | | March 30, 2017 | | 001-37471 |
| | | | | | | | | |
| | 2018 Employee, Director and Consultant Equity Incentive Plan | # | | Form 8-K (Exhibit 10.1) | | July 26, 2018 | | 001-37471 |
| | | | | | | | | |
| | Form of Stock Option Award Agreement under the Registrant’s 2018 Employee, Director and Consultant Equity Incentive Plan | # | | Form S-8 (Exhibit 10.2) | | August 9, 2018 | | 333-226733 |
| | 2018 Employee Stock Purchase Plan | # | | Form 8-K (Exhibit 10.2) | | July 26, 2018 | | 001-37471 |
|
| | | | | | | | | |
Exhibit Number | | Exhibit Description | | | Incorporated by Reference herein from Form or Schedule | | Filing Date | | SEC File / Registration Number |
| | Research and Licensing Agreement by and between Pieris AG and Technische Universität München, dated as of July 26, 2007 | ± | | Form 10-K (Exhibit 10.10) | | March 30, 2015 | | 333-190728 |
| | | | | | |
| | | | | | | | | |
| | License and Transfer Agreement by and between the Company and Enumeral Biomedical Holdings, Inc dated as of April 18, 2016 | ± | | Form 10-Q/A (Exhibit 10.1) | | July 20, 2016 | | 001-37471 |
| | | | | | |
| | Definitive License and Transfer Agreement by and between the Company and Enumeral Biomedical Holdings, Inc. dated as of June 6, 2016 | ± | | Form 10-Q (Exhibit 10.1) | | August 11, 2016 | | 001-37471 |
| | | | | |
| | Amendment No.1 to Definitive License and Transfer Agreement by and between the Company and Enumeral Biomedical Holdings, Inc. effective as of January 3, 2017 | | | Form 10-K (Exhibit 10.14) | | March 30, 2017 | | 001-37471 |
| | | | | | |
| | Collaboration Agreement by and among the Registrant, Pieris Pharmaceuticals GmbH, Les Laboratoires Servier and Institut de Recherches Internationales Servier, dated as of January 4, 2017 | ±
| | Form 10-K/A (Exhibit 10.15) | | April 26, 2018 | | 001-37471 |
| | | | | | |
| | Non-Exclusive Anticalin Platform Technology License Agreement by and among the Registrant, Pieris Pharmaceuticals GmbH, Les Laboratoires Servier and Institut de Recherches Internationales Servier, dated as of January 4, 2017 | ±
| | Form 10-K/A (Exhibit 10.16) | | April 26, 2018 | | 001-37471 |
| | | | | | |
| | First Amendment to the License and Collaboration Agreement by and between Les Laboratoires Servier, Institut de Recherches Internationales Servier, Pieris Pharmaceuticals, Inc. and Pieris Pharmaceuticals GmbH, effective as of June 16, 2017 | ±
| | Form 10-Q/A (Exhibit 10.4) | | April 26, 2018 | | 001-37471 |
| | | | | | | | | |
| | Exclusive Option Agreement by and among the Registrant, Pieris Pharmaceuticals GmbH and ASKA Pharmaceutical Co., Ltd., dated as of February 27, 2017 | ±
| | Form 10-Q/A (Exhibit 10.3) | | April 26, 2018
| | 001-37471 |
| | | | | | | | | |
| | License & Collaboration Agreement by and between Pieris Pharmaceuticals Inc., Pieris Pharmaceuticals GmbH & Pieris Australia Pty. Limited and AstraZeneca AB, dated as of May 2, 2017 | ±
| | Form 10-Q/A (Exhibit 10.1) | | April 26, 2018
| | 001-37471 |
| | | | | | | | | |
| | Non-Exclusive Anticalin® Platform Technology License Agreement, by and between Pieris Pharmaceuticals Inc., Pieris Pharmaceuticals GmbH and Pieris Australia Pty. Limited and AstraZeneca AB, dated as of May 2, 2017 | ±
| | Form 10-Q/A (Exhibit 10.2) | | April 26, 2018 | | 001-37471 |
| | | | | | | | | |
| | License and Collaboration Agreement by and among the Registrant, Pieris GmbH and Seattle Genetics, Inc., dated February 8, 2018
| ± | | Form 10-Q (Exhibit 10.1)
| | May 10, 2018 | | 001-37471 |
| | | | | | | | | |
|
| | | | | | | | | |
Exhibit Number | | Exhibit Description | | | Incorporated by Reference herein from Form or Schedule | | Filing Date | | SEC File / Registration Number |
| | Non-Exclusive Anticalin Platform Technology License Agreement by and among the Registrant, Pieris Pharmaceuticals GmbH and Seattle Genetics, Inc., dated February 8, 2018
| ± | | Form 10-Q (Exhibit 10.2) | | May 10, 2018 | | 001-37471 |
| | | | | | | | | |
| | Form of Indemnification Agreement by and between the Registrant and each of its current directors and executive officers | # | | Form 8-K (Exhibit 10.10) | | December 18, 2014 | | 333-190728 |
| | | | | |
| | Employment Agreement by and between the Registrant and Stephen S. Yoder, dated as of December 17, 2014 | # | | Form 8-K (Exhibit 10.15) | | December 18, 2014 | | 333-190728 |
| | | | | |
| | Separation Agreement by and between the Registrant and Darlene Deptula-Hicks, dated as of February 7, 2017 | # | | Form 10-K (Exhibit 10.26) | | March 30, 2017 | | 001-37471 |
| | | | | |
| | Employment Agreement by and between the Registrant and Louis A. Matis, M.D., dated as of July 20, 2015 | # | | Form 10-Q (Exhibit 10.1) | | November 13, 2015 | | 001-37471 |
| | | | | |
| | Consulting Agreement by and between the Registrant and Danforth Advisors, LLC, dated as of February 1, 2017 | # | | Form 10-K (Exhibit 10.29) | | March 30, 2017 | | 001-37471 |
| | | | | |
| | Employment Agreement by and between Pieris Pharmaceuticals, Inc. and Allan Reine, dated as of August 9, 2017 | # | | Form 10-Q (Exhibit 10.5) | | August 11, 2017 | | 001-37471 |
| | | | | | | | | |
| | Non-Qualified Stock Option Agreement, dated August 9, 2017, between Pieris Pharmaceuticals Inc. and Allan Reine, M.D. | # | | Form 10-Q (Exhibit 10.2) | | November 13, 2017 | | 001-37471 |
| | | | | | | | | |
| | Non-Employee Director Compensation Policy, as amended | *# | | | | | | |
| | | | | |
| | Lease Agreement by and between Pieris AG and Födergesellschft IZB mbH, dated as of May 4, 2011 | | | Form 8-K (Exhibit 10.23) | | December 18, 2014 | | 333-190728 |
| | | | | |
| | Agreement of Sublease by and between Berenberg Capital Markets LLC and the Registrant, dated as of August 27, 2015 | | | Form 10-Q (Exhibit 10.3) | | November 13, 2015 | | 001-37471 |
| | | | | |
| | Lease Agreement by and between Pieris GmbH and Hallbergmoos Grundvermögen GmbH, dated October 24, 2018 | * | | | | | | |
| | | | | | | | | |
| | Repayment Agreement by and between Pieris AG and tbg Technologie-Beteiligungs-Gesellschaft mbH, dated as of April 3, 2014 | | | Form 8-K (Exhibit 10.27) | | December 18, 2014 | | 333-190728 |
| | | | | | | | | |
| | Settlement Agreement (Accelerated Repayment Agreement) by and between Pieris AG and tbg Technologie-Beteiligungs-Gesellschaft mbH, dated as of December 11, 2014 | | | Form 8-K (Exhibit 10.28) | | December 18, 2014 | | 333-190728 |
| | | | | |
| | Consolidated Shareholders’ Agreement 2014, Pieris AG, Freising, Germany, by and among Pieris AG and the Stockholders party thereto, dated October 10, 2014 | | | Form 8-K (Exhibit 10.30) | | December 18, 2014 | | 333-190728 |
| | | | | |
|
| | | | | | | | | |
Exhibit Number | | Exhibit Description | | | Incorporated by Reference herein from Form or Schedule | | Filing Date | | SEC File / Registration Number |
| | Investment Agreement, Pieris AG, Freising, Germany, by and among Pieris AG, Stephen Yoder and the Existing Shareholders party thereto, dated October 10, 2014 | | | Form 8-K (Exhibit 10.31) | | December 18, 2014 | | 333-190728 |
| | | | | |
| | Agreement, by and among Pieris AG and the Stockholders party thereto, dated December 5, 2014 | | | Form 8-K (Exhibit 10.32) | | December 18, 2014 | | 333-190728 |
| | | | | |
| | Form of Securities Purchase Agreement, dated December 17, 2014, by and among the Registrant and the Purchasers | | | Form 8-K (Exhibit 10.1) | | December 23, 2014 | | 333-190728 |
| | | | | |
| | Form of Registration Rights Agreement, dated December 17, 2014, by and among the Registrant and the investors party thereto | | | Form 8-K (Exhibit 10.2) | | December 23, 2014 | | 333-190728 |
| | | | | |
| | Form of Warrant to Purchase Common Stock, dated December 17, 2014, issued by the Registrant | | | Form 8-K (Exhibit 10.3) | | December 23, 2014 | | 333-190728 |
| | | | | |
| | Securities Purchase Agreement, dated June 2, 2016, by and among the Registrant and the Investors named therein | | | Form 8-K (Exhibit 10.1) | | June 6, 2016 | | 001-37471 |
| | | | | |
| | Form of Warrant to purchase Common Stock, dated June 2, 2016, issued by the Registrant | | | Form 8-K (Exhibit 10.2) | | June 6, 2016 | | 001-37471 |
| | | | | |
| | Registration Rights Agreement, dated June 2, 2016, by and among the Registrant and the Investors named therein | | | Form 8-K (Exhibit 10.3) | | June 6, 2016 | | 001-37471 |
| | | | | |
| | Exchange Agreement, dated January 30, 2019, by and among the Registrant and Biotechnology Value Fund, L.P., Biotechnology Value Fund II, L.P., and Biotechnology Value Trading Fund OS, L.P. | | | Form 8-K (Exhibit 10.1) | | February 4, 2019 | | 001-37471 |
| | Corporate Code of Ethics and Conduct and Whistleblower Policy | * | | | | | | |
| | | | | |
| | List of Subsidiaries | * | | | | | | |
| | | | | |
| | Consent of Ernst & Young LLP | * | | | | | | |
| | | | | |
| | Certification of Stephen S. Yoder, Chief Executive Officer and President, pursuant to Section 302 of the Sarbanes—Oxley Act of 2002 | * | | | | | | |
| | | | | |
| | Certification of Allan Reine, Chief Financial Officer, pursuant to Section 302 of the Sarbanes—Oxley Act of 2002 | * | | | | | | |
| | | | | | | | | |
| | Certification of Stephen S. Yoder, Chief Executive Officer and President, pursuant to Section 906 of the Sarbanes—Oxley Act of 2002, 18 U.S.C. Section 1350 | ** | | | | | | |
| | | | | |
| | Certification of Allan Reine, Chief Financial Officer, pursuant to Section 906 of the Sarbanes—Oxley Act of 2002, 18 U.S.C. Section 1350 | ** | | | | | | |
| | | | | |
101.INS | | XBRL Instance Document | * | | | | | | |
| | | | | |
101.SCH | | XBRL Taxonomy Extension Schema Document | * | | | | | | |
| | | | | |
|
| | | | | | | | | |
Exhibit Number | | Exhibit Description | | | Incorporated by Reference herein from Form or Schedule | | Filing Date | | SEC File / Registration Number |
101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document | * | | | | | | |
| | | | | |
101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document | * | | | | | | |
| | | | | |
101.LAB | | XBRL Taxonomy Extension Label Linkbase Document | * | | | | | | |
| | | | | |
101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document | * | | | | | | |
| | | | | | | | | |
* | Filed herewith | | | | | | | |
| | | | | | | | | |
** | Furnished herewith | | | | | | | |
| | | | | | | | | |
± | Confidential treatment received as to portions of the exhibit. Confidential materials omitted and filed separately with the SEC. | | | | | | | |
| | | | | | | | | |
# | Indicates a management contract or compensatory plan | | | | | | | |
|
| | |
Item 16. | | FORM 10-K SUMMARY |
We may voluntarily include a summary of information required by Form 10-K under this Item 16. We have elected not to include such summary information.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | | |
| PIERIS PHARMACEUTICALS, INC. |
| | | |
March 18, 2019 | By: | | /s/ Stephen S. Yoder |
| | | Stephen S. Yoder |
| | | Chief Executive Officer and President |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
|
| | | | |
Signature | | Title | | Date |
| | |
/s/ Stephen S. Yoder | | President, Chief Executive Officer and | | March 18, 2019 |
Stephen S. Yoder | | Director (Principal Executive Officer) | | |
| | |
/s/ Allan Reine, M.D. | | Chief Financial Officer and | | March 18, 2019 |
Allan Reine, M.D. | | Treasurer (Principal Financial and Accounting Officer) | | |
| | |
/s/ James Geraghty | | Chairman of the Board of Directors | | March 18, 2019 |
James Geraghty | | | | |
| | |
/s/ Jean-Pierre Bizzari, M.D. | | Director | | March 18, 2019 |
Jean-Pierre Bizzari, M.D. | | | | |
| | |
/s/ Michael Richman | | Director | | March 18, 2019 |
Michael Richman | | | | |
| | |
/s/ Steven Prelack | | Director | | March 18, 2019 |
Steven Prelack | | | | |
| | |
/s/ Peter Kiener, D.Phil. | | Director | | March 18, 2019 |
Peter Kiener, D.Phil. | | | | |
| | |
/s/ Christopher Kiritsy | | Director | | March 18, 2019 |
Christopher Kiritsy | | | | |
| | | | |
/s/ Ann Barbier, M.D., Ph.D. | | Director | | March 18, 2019 |
Ann Barbier, M.D., Ph.D. | | | | |
| | | | |
/s/ Matthew L. Sherman, M.D. | | Director | | March 18, 2019 |
Matthew L. Sherman, M.D. | | | | |
PIERIS PHARMACEUTICALS, INC.
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Pieris Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Pieris Pharmaceuticals, Inc. (the Company) as of December 31, 2018 and 2017, the related consolidated statements of operations, comprehensive loss, stockholders' equity, and cash flows for each of the two years in the period ended December 31, 2018, and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2018, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company´s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
|
| | | | |
/s/ Ernst & Young LLP | | | | |
We have served as the Company's auditor since 2016. | | | | |
Boston, Massachusetts | | | | |
March 18, 2019 | | | | |
PIERIS PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
|
| | | | | | | | |
| | December 31, |
| | 2018 | | 2017 |
Assets | | | | |
Current assets: | | | | |
Cash and cash equivalents | | $ | 74,867 |
| | $ | 37,878 |
|
Short term investments | | 53,240 |
| | 34,751 |
|
Accounts receivable | | 2,701 |
| | 15,546 |
|
Prepaid expenses and other current assets | | 4,574 |
| | 1,615 |
|
Total current assets | | 135,382 |
| | 89,790 |
|
Property and equipment, net | | 5,049 |
| | 4,034 |
|
Long term investments | | — |
| | 9,922 |
|
Other non-current assets | | 910 |
| | 130 |
|
Total assets | | $ | 141,341 |
| | $ | 103,876 |
|
Liabilities and stockholders’ equity | | | | |
Current liabilities: | | | | |
Accounts payable | | $ | 3,350 |
| | $ | 2,452 |
|
Accrued expenses and other current liabilities | | 9,114 |
| | 6,170 |
|
Deferred revenues, current portion | | 35,612 |
| | 37,153 |
|
Total current liabilities | | 48,076 |
| | 45,775 |
|
Deferred revenue, net of current portion | | 53,303 |
| | 46,542 |
|
Other long-term liabilities | | 27 |
| | 37 |
|
Total liabilities | | 101,406 |
| | 92,354 |
|
Stockholders’ equity: | | | | |
Preferred stock, $0.001 par value per share, 10,000 shares authorized and 2,907 and 4,963 issued and outstanding at December 31, 2018 and 2017, respectively | | — |
| | — |
|
Common stock, $0.001 par value per share, 300,000,000 shares authorized and 54,151,219 and 45,017,062 issued and outstanding at December 31, 2018 and 2017, respectively | | 54 |
| | 45 |
|
Additional paid-in capital | | 189,929 |
| | 136,484 |
|
Accumulated other comprehensive loss | | (2,982 | ) | | (4,695 | ) |
Accumulated deficit | | (147,066 | ) | | (120,312 | ) |
Total stockholders’ equity | | 39,935 |
| | 11,522 |
|
Total liabilities and stockholders’ equity | | $ | 141,341 |
| | $ | 103,876 |
|
The accompanying notes are an integral part of these consolidated financial statements.
PIERIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except per share data)
|
| | | | | | | | |
| | Years Ended December 31, |
| | 2018 | | 2017 |
Revenue | | $ | 29,101 |
| | $ | 25,275 |
|
Operating expenses | | | | |
Research and development | | 41,490 |
| | 22,285 |
|
General and administrative | | 18,442 |
| | 17,584 |
|
Total operating expenses | | 59,932 |
| | 39,869 |
|
Loss from operations | | (30,831 | ) | | (14,594 | ) |
Interest income | | 1,962 |
| | 152 |
|
Other income (expense), net | | 1,803 |
| | (2,102 | ) |
Loss before income taxes | | (27,066 | ) | | (16,544 | ) |
(Benefit) provision for income tax | | (312 | ) | | 1,103 |
|
Net loss | | (26,754 | ) | | (17,647 | ) |
| | | | |
Foreign currency translation | | 1,196 |
|
| (2,374 | ) |
Unrealized gain (loss) on available-for-sale securities, net of tax provision of $164 and $0, respectively | | 517 |
| | (820 | ) |
Comprehensive loss | | $ | (25,041 | ) | | $ | (20,841 | ) |
Net loss per share | | | | |
Basic and diluted | | $ | (0.50 | ) | | $ | (0.40 | ) |
Weighted average number of common shares outstanding | | | | |
Basic and diluted | | 53,081 |
| | 43,931 |
|
The accompanying notes are an integral part of these consolidated financial statements.
PIERIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(In thousands)
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Convertible series A preferred shares | | Common Shares | | Additional paid-in capital | | Accumulated other comprehensive loss | | Accumulated deficit | | Total equity |
| | No. of shares | | Share capital | | No. of shares | | Share capital | |
Balance as of January 1, 2017 | | 5 |
| | $ | — |
| | 43,059 |
| | $ | 43 |
| | $ | 129,350 |
| | $ | (1,501 | ) | | $ | (102,665 | ) | | $ | 25,227 |
|
Net loss | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (17,647 | ) | | (17,647 | ) |
Foreign currency translation adjustment | | — |
| | — |
| | — |
| | — |
| | — |
| | (2,374 | ) | | — |
| | (2,374 | ) |
Unrealized losses on investments | | — |
| | — |
| | — |
| | — |
| | — |
| | (820 | ) | | — |
| | (820 | ) |
Stock based compensation expense | | — |
| | — |
| | — |
| | — |
| | 3,046 |
| | — |
| | — |
| | 3,046 |
|
Issuance of common stock resulting from exercise of stock options | | — |
| | — |
| | 453 |
| | — |
| | 781 |
| | — |
| | — |
| | 781 |
|
Issuance of common stock resulting from exercise of warrants | | — |
| | — |
| | 1,505 |
| | 2 |
| | 3,307 |
| | — |
| | — |
| | 3,309 |
|
Balance as of December 31, 2017 | | 5 |
| | — |
| | 45,017 |
| | 45 |
| | 136,484 |
| | (4,695 | ) | | (120,312 | ) | | 11,522 |
|
Net loss | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (26,754 | ) | | (26,754 | ) |
Foreign currency translation adjustment | | — |
| | — |
| | — |
| | — |
| | — |
| | 1,196 |
| | — |
| | 1,196 |
|
Unrealized gain on investments, net of $164 tax provision | | — |
| | — |
| | — |
| | — |
| | — |
| | 517 |
| | — |
| | 517 |
|
Stock based compensation expense | | — |
| | — |
| | — |
| | — |
| | 4,943 |
| | — |
| | — |
| | 4,943 |
|
Issuance of common stock resulting from exercise of stock options | | — |
| | — |
| | 596 |
| | 1 |
| | 985 |
| | — |
| | — |
| | 986 |
|
Issuance of common stock resulting from exercise of warrants | | — |
| | — |
| | 157 |
| | — |
| | 314 |
| | — |
| | — |
| | 314 |
|
Issuance of common stock net $3,374 in offering costs | | — |
| | | | 6,325 |
| | 6 |
| | 47,205 |
| | — |
| | — |
| | 47,211 |
|
Preferred stock conversion | | (2 | ) | | — |
| | 2,056 |
| | 2 |
| | (2 | ) | | — |
| | — |
| | — |
|
Balance as of December 31, 2018 | | 3 |
| | $ | — |
| | 54,151 |
| | $ | 54 |
| | $ | 189,929 |
| | $ | (2,982 | ) | | $ | (147,066 | ) | | $ | 39,935 |
|
The accompanying notes are an integral part of these consolidated financial statements.
PIERIS PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
| | | | | | | | |
| | Years Ended December 31, |
| | 2018 | | 2017 |
Operating activities: | |
| |
|
Net loss | | $ | (26,754 | ) | | $ | (17,647 | ) |
Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | |
| |
|
Depreciation | | 570 |
| | 369 |
|
Stock-based compensation | | 4,943 |
| | 3,046 |
|
Other non-cash transactions | | 75 |
| | 293 |
|
Realized investment gains | | (1,651 | ) | | — |
|
Deferred tax benefit | | (164 | ) | | — |
|
Foreign currency re-measurement loss |
| 22 |
|
| 156 |
|
Changes in operating assets and liabilities: | |
| |
|
Accounts receivable | | 12,511 |
| | (14,469 | ) |
Prepaid expenses and other assets | | (3,939 | ) | | 1,900 |
|
Deferred revenue | | 9,308 |
| | 74,378 |
|
Accounts payable | | 764 |
| | (332 | ) |
Accrued expenses and other current liabilities | | 3,249 |
| | 2,060 |
|
Net cash (used in)/provided by operating activities | | (1,066 | ) | | 49,754 |
|
Investing activities: | |
| |
|
Purchase of property and equipment | | (1,698 | ) | | (1,950 | ) |
Proceeds from maturities of investments | | 88,358 |
| | — |
|
Proceeds from sale of investments | | 22,047 |
| | — |
|
Purchase of investments | | (117,582 | ) | | (43,925 | ) |
Net cash used in investing activities | | (8,875 | ) | | (45,875 | ) |
Financing activities: | |
| |
|
Proceeds from exercise of options | | 986 |
| | 781 |
|
Proceeds from exercise of warrants | | 314 |
| | 3,309 |
|
Issuance of Common and Preferred Stock, net of issuance costs | | 47,211 |
| | — |
|
Net cash provided by financing activities | | 48,511 |
| | 4,090 |
|
Effect of exchange rate change on cash and cash equivalents | | (1,581 | ) | | 553 |
|
Net increase in cash and cash equivalents | | 36,989 |
| | 8,522 |
|
Cash and cash equivalents at beginning of year | | 37,878 |
| | 29,356 |
|
Cash and cash equivalents at end of year | | $ | 74,867 |
| | $ | 37,878 |
|
Supplemental cash flow disclosures: | |
| |
|
Cash paid for income taxes | | $ | 908 |
| | $ | — |
|
Net unrealized gain (loss) on investments | | $ | 681 |
| | $ | (820 | ) |
Property and equipment included in accounts payable | | $ | 241 |
| | $ | 114 |
|
The accompanying notes are an integral part of these consolidated financial statements.
PIERIS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Corporate Information
Pieris Pharmaceuticals, Inc., was founded in May 2013, and acquired 100% interest in Pieris GmbH in December 2014. Pieris is a clinical-stage biopharmaceutical company that discovers and develops Anticalin-based drugs to target validated disease pathways in unique and transformative ways. Pieris's corporate headquarters is located in Boston, Massachusetts and its research facility is located in Freising-Weihenstephan, Germany.
Pieris's clinical pipeline includes an immuno-oncology bispecific targeting HER2 and 4-1BB, an inhaled IL-4Rα antagonist Anticalin protein to treat uncontrolled asthma, and a half-life-optimized hepcidin antagonizing Anticalin protein to treat anemia.
The Company’s core Anticalin technology and platform was developed in Germany, and the Company has partnership arrangements with several major multi-national pharmaceutical companies.
As of December 31, 2018, the Company had cash, cash equivalents and investments of $128.1 million. The Company expects that its existing cash, cash equivalents, and investments, are sufficient to support operating expenses and capital expenditure requirements for at least 12 months from the date of filing.
2. Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The accompanying consolidated financial statements of the Company and its wholly-owned subsidiaries were prepared in accordance with GAAP. The consolidated financial statements include the accounts of all subsidiaries. All intercompany balances and transactions have been eliminated.
The preparation of the financial statements in accordance with GAAP requires management to make estimates, judgments, and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses and the related disclosures at the date of the financial statements and during the reporting period. Significant estimates are used for, but are not limited to, revenue recognition; deferred tax assets, deferred tax liabilities and valuation allowances; fair value of stock options and various accruals. Management evaluates its estimates on an ongoing basis. Actual results and outcomes could differ materially from management’s estimates, judgments, and assumptions.
Foreign Currency Translation
The financial statements of the Company’s foreign subsidiaries are translated from local currency into reporting currency, which is US dollars, using the current exchange rate at the balance sheet date for assets and liabilities, and the weighted average exchange rate prevailing during the period for revenues and expenses. The functional currency for Pieris’ foreign subsidiaries is considered to be the local currency for each entity and, accordingly, translation adjustments for these subsidiaries are included in accumulated other comprehensive loss within stockholders’ equity.
Realized and unrealized gains and losses resulting from foreign currency transactions denominated in currencies other than the functional currency are reflected as other (expense) income, net in the consolidated statements of operations. Foreign currency gains and losses on available-for-sale investment transactions are recorded to other comprehensive income on the Company's balance sheet per Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Topic 830, Foreign Currency Matters.
Cash, Cash Equivalents and Investments
The Company determines the appropriate classification of its investments at the time of purchase. All liquid investments with original maturities of 90 days or less from the purchase date are considered to be cash equivalents. The Company’s current and non-current investments are comprised of money market, asset backed securities, government treasuries, and corporate bonds that are classified as available-for-sale in accordance with FASB ASC Topic 320, Investments—Debt and Equity Securities. The Company classifies investments available to fund current operations as current assets on its balance sheets. Investments are classified as non-current assets on the balance sheets if (i) the Company has the intent and ability to hold the investments for a period of at least one year and (ii) the contractual maturity date of the investments is greater than one year.
Available-for-sale investments are recorded at fair value, with unrealized gains or losses included in accumulated other comprehensive loss on the Company’s balance sheets. Realized gains and losses are determined using the specific identification method and are included as a component of other income.
The Company reviews investments for other-than-temporary impairment whenever the fair value of an investment is less than the amortized cost and evidence indicates that an investment’s carrying amount is not recoverable within a reasonable period of time. To determine whether an impairment is other-than temporary, the Company considers its intent to sell, or whether it is more likely than not that the Company will be required to sell the investment before recovery of the investment’s amortized cost basis. Evidence considered in this assessment includes reasons for the impairment, the severity and the duration of the impairment, and changes in value subsequent to period end. As of December 31, 2018, there were no investments with a fair value that was significantly lower than the amortized cost basis or any investments that had been in an unrealized loss position for a significant period.
Concentration of Credit Risk and Off-Balance Sheet Risk
The Company has no financial instruments with off‑balance sheet risk such as foreign exchange contracts, option contracts, or other foreign hedging arrangements. Financial instruments that subject the Company to concentrations of credit risk include cash and cash equivalents, investments, and accounts receivable. The Company’s cash, cash equivalents, and investments are held in accounts with financial institutions that management believes are creditworthy. The Company’s investment policy includes guidelines on the quality of the institutions and financial instruments and defines allowable investments that the Company believes minimizes the exposure to concentration of credit risk. These amounts, at times, may exceed federally insured limits. The Company has not experienced any credit losses in such accounts and does not believe it is exposed to any significant credit risk on these funds. Accounts receivable primarily consist of amounts due under strategic partnership and other license agreements with major multi-national pharmaceutical companies for which the Company does not obtain collateral.
Fair Value Measurement
The Company is required to disclose information on all assets and liabilities reported at fair value that enables an assessment of the inputs used in determining the reported fair values. FASB ASC Topic 820, Fair Value Measurement and Disclosures, or ASC 820, established a hierarchy of inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the financial instrument based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the financial instrument and are developed based on the best information available in the circumstances. The fair value hierarchy applies only to the valuation inputs used in determining the reported or disclosed fair value of the financial instruments and is not a measure of the investment credit quality. Fair value measurements are classified and disclosed in one of the following three categories:
| |
• | Level 1 inputs are quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. |
| |
• | Level 2 utilizes quoted market prices in markets that are not active, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency. |
| |
• | Level 3 inputs are unobservable inputs for the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date. |
To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
Financial instruments measured at fair value on a recurring basis include cash equivalents and investments (Note 4).
An entity may elect to measure many financial instruments and certain other items at fair value at specified election dates. Subsequent unrealized gains and losses on items for which the fair value option has been elected will be reported in net loss. The Company did not elect to measure any additional financial instruments or other items at fair value.
Fair Values of Financial Instruments
The fair value of cash, accounts receivable, and accounts payable approximates the carrying value of these financial instruments because of the short-term nature of any maturities. The Company determines the estimated fair values of other financial instruments, using available market information and valuation methodologies, primarily input from independent third party pricing sources.
Accounts Receivable
Accounts receivable are recorded net of allowances for doubtful accounts and represent amounts due from third parties, strategic partners, and other license agreement counterparties. The Company monitors and evaluates collectability of receivables on an ongoing basis and considers whether an allowance for doubtful accounts is necessary. The Company determined that no such reserve is needed as of December 31, 2018 and 2017. Historically, Pieris has not had collectability issues.
Property and Equipment
Property and equipment are recorded at acquisition cost, less accumulated depreciation and impairment. Depreciation on property and equipment is calculated using the straight-line method over the remaining estimated useful lives of the assets. Maintenance and repairs to these assets are charged to expenses as occurred. The estimated useful life of the different groups of property and equipment is as follows:
|
| | |
Asset Classification | | Estimated useful life (in years) |
Leasehold improvements | | shorter of useful life or remaining life of the lease |
Laboratory equipment | | 10 - 14 |
Office and computer equipment | | 3 - 13 |
Impairment of Long-lived Assets
The Company reviews its long-lived assets to be held and used for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. The Company evaluates the realizability of its long-lived assets based on profitability and cash flow expectations for the related asset. Any write-downs are treated as permanent reductions in the carrying amount of the assets. The Company believes that, as of each of the balance sheets presented, none of the Company’s long-lived assets were impaired.
Revenue Recognition
Pieris has entered into several licensing agreements with collaboration partners for the development of Anticalin therapeutics against a variety of targets in diseases and conditions. The terms of these agreements contain multiple elements and deliverables, which may include: (i) licenses, or options to obtain licenses, to Pieris’s Anticalin technology and/or specific programs and (ii) research and development activities to be performed on behalf of or with the collaborative partner. Payments to Pieris under these agreements may include upfront fees (which include license and option fees), payments for research and development activities, payments based upon the achievement of certain milestones, and royalties on product sales. There are no performance, cancellation, termination or refund provisions in any of the arrangements that could result in material financial consequences to Pieris. Pieris follows the provisions of the FASB ASC Topic 605-25, Revenue Recognition—Multiple-Element Arrangements, or ASC 605-25, and FASB ASC Topic 605-28, Revenue Recognition—Milestone Method, or ASC 605-28, in accounting for these agreements.
Multiple-Element Arrangements
When evaluating multiple-element arrangements, Pieris identifies the deliverables included within the agreement and evaluates which deliverables represent separate units of accounting based on whether the delivered element has stand-alone value to the customer or if the arrangement includes a general right of return for delivered items.
The consideration received is allocated among the separate units of accounting using the relative selling price method, and the applicable revenue recognition criteria are applied to each of the separate units of accounting. Pieris uses the BESP methodology to estimate the selling price for each deliverable and unit of accounting because Pieris does not have VSOE or TPE of selling price for these deliverables. To determine the estimated selling price of a deliverable, Pieris considers market conditions as well as entity-specific factors, including those factors contemplated in negotiating the agreements, terms of previous collaborative agreements, similar agreements entered into by third parties, market opportunity, estimated development
costs, probability of success, and the time needed to commercialize a product candidate pursuant to the license. In validating Pieris’s BESP, Pieris evaluates whether changes in the key assumptions used to determine the BESP will have a significant effect on the allocation of arrangement consideration among multiple deliverables.
Multiple element arrangements, such as license arrangements, are analyzed to determine whether the deliverables, which often include licenses and performance obligations such as research and development services and governance committee services, can be separated or whether they must be accounted for as a combined unit of accounting in accordance with GAAP. The Company recognizes the arrangement consideration allocated to licenses as revenue upon delivery of the license only if the license has stand-alone value. If the license is considered not to have stand-alone value, the license would then be combined with other undelivered elements into a combined unit of accounting and the license payments and payments for performance obligations would be recognized as revenue when the revenue recognition criteria have been satisfied for the last deliverable within the unit of accounting. In the case of combined units of accounting that include delivered licenses and undelivered services to be provided over time, revenue would be recognized over the estimated period during which services will be provided. For units of accounting that include licenses to be delivered upon satisfactory completion of certain research services, revenue is deferred until the license is delivered and the performance obligation is satisfied.
If the Company is involved in a governance committee, as part of a multiple element arrangement, it assesses whether its involvement constitutes a performance obligation or a right to participate. When governance committee services are determined to be performance obligations, the Company determines the fair value to be allocated to this deliverable and recognize the revenue over the expected term of the development period of the products. Otherwise, the fair value for participation is combined with other research services or performance obligations and is recognized over the term which the Company expects to complete its aggregate performance obligations.
The Company recognizes arrangement consideration allocated to each unit of accounting when all revenue recognition criteria in SEC Staff Accounting Bulletin No. 104, "Revenue Recognition in Financial Statements," or SAB 104, are satisfied for that particular unit of accounting. For each unit of accounting, the Company must determine the period over which the performance obligations will be performed and revenue will be recognized. If there is no discernible pattern of performance or objectively measurable performance measures do not exist, then the Company recognizes revenue under the arrangement on a straight-line basis over the period the Company is expected to complete its performance obligations. Conversely, if the pattern of performance over which the service is provided to the customer can be determined and objectively measurable performance measures exist, then the Company recognizes revenue under the arrangement using the proportional performance method. Revenue recognized cannot exceed the amount that has been earned and has been billed or is currently billable.
Significant management judgment is required in determining the level of effort required under an arrangement and the period over which the Company is expected to complete its performance obligations under an arrangement.
The accounting treatment for options granted to collaborators is dependent upon the nature of the option granted to the collaborative partner. Options are considered substantive if, at the inception of an agreement, Pieris is at risk as to whether the collaborative partner will choose to exercise the option(s) to secure additional goods or services. Factors that are considered in evaluating whether options are substantive include the overall objective of the arrangement, benefit the collaborator might obtain from the agreement without exercising the options, cost to exercise the options relative to the total upfront consideration, and additional financial commitments or economic penalties imposed on the collaborator as a result of exercising the options.
In arrangements where options to obtain additional deliverables are considered substantive, Pieris determines whether the optional licenses are priced at a significant and incremental discount. If the prices include a significant and incremental discount, the option is considered a deliverable in the arrangement. However, if not priced at a significant and incremental discount, the option is not considered a deliverable in the arrangement. When a collaborator exercises an option considered to be at a significant and incremental discount to acquire an additional license, the exercise fee that is attributed to the additional license and any incremental discount allocated at inception are recognized in a manner consistent with the treatment of up-front payments for licenses (i.e., license and research services). In the event an option expires un-exercised, any incremental discounts deferred at the inception of the arrangement are recognized into revenue upon expiration. For options that are non-substantive, the additional licenses to which the options pertain are considered deliverables upon inception of the arrangement; Pieris applies the multiple-element revenue recognition criteria to determine accounting treatment.
Payments or reimbursements resulting from Pieris’s research and development efforts in multi-element arrangements, in which Pieris’s research and development efforts are considered to be a deliverable, are included in allocable consideration and allocated to the units of accounting. These reimbursements are recognized as the services are performed and are presented on a gross basis, so long as there is persuasive evidence of an arrangement, the fee is fixed or determinable, and collection of the related receivable is reasonably assured. Revenue recognized cannot exceed the amount that has been earned and has been
billed or is currently billable. Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue in the accompanying balance sheets.
Milestone Payments and Royalties
At the inception of each agreement that includes milestone payments, Pieris evaluates whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. This evaluation includes an assessment of whether: (a) the consideration is commensurate with either (1) the entity’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity’s performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. Pieris evaluates factors such as the scientific, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required to achieve the respective milestone and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment.
The Company aggregates milestones into four categories (i) research milestones, (ii) development milestones, (iii) commercial milestones and (iv) sales milestones. Research milestones are typically achieved upon reaching certain success criteria as defined in each agreement related to developing an Anticalin protein against the specified target. Development milestones are typically reached when a compound reaches a defined phase of clinical research or passes such phase, or upon gaining regulatory approvals. Commercial milestones are typically achieved when an approved pharmaceutical product reaches the status for commercial sale, including regulatory approval. Sales milestones are certain defined levels of net sales by the licensee, such as when a product first achieves global sales or annual sales of a specified amount.
For revenues from research, development, and commercial milestone payments, if the milestones are deemed substantive and the milestone payments are nonrefundable, such amounts are recognized entirely upon successful accomplishment of the milestones, assuming all other revenue recognition criteria are met. Milestones that are not considered substantive are accounted for as contingent revenue and will be recognized when achieved to the extent the Company has no remaining performance obligations under the arrangement. Revenues from sales milestone payments are accounted for as royalties and are recorded as revenue upon achievement of the milestone, assuming all other revenue recognition criteria are met. Royalty payments are recognized in revenues based on the timing of royalty payments earned in accordance with the agreements, which typically is the period when the relevant sales occur, assuming all other revenue recognition criteria are met.
Research and Development
Research and development expenses are charged to the statement of operations as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including salaries and benefits, facilities costs, pre-clinical and clinical costs, contract services, consulting, depreciation and amortization expense, and other related costs. Costs associated with acquired technology, in the form of upfront fees or milestone payments, are charged to research and development expense as incurred.
Incremental Direct Costs
Incremental direct costs incurred related to the acquisition or origination of a customer contract in a transaction that results in the deferral of revenue are recorded to the statement of operations as incurred.
Income Taxes
The Company applies ASC Topic 740 Income Taxes, which established financial accounting and reporting requirements for the effects of income taxes that result from the Company’s activities during the current and preceding years. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating losses and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted statutory tax rates expected to apply to taxable income in the jurisdictions and years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Where the Company determines that it is more likely than not that some portion or all of the deferred tax assets will not be realized in the future, the deferred tax assets are reduced by a valuation allowance. The Company records interest related and penalties related to uncertain tax positions as part of income tax expense.
Stock-based Compensation
The Company measures share-based payments in accordance with ASC Topic 718, Stock Compensation. Pieris records its stock-based compensation expense over the requisite service period and records forfeitures as they occur. Determining the appropriate fair value model and related assumptions requires judgment, including estimating share price volatility and expected terms of the awards. For employee options, the fair value measurement date is generally on the date of grant and the related compensation expense is recognized on a straight-line basis over the requisite period of the awards, less expense for actual forfeitures.
The Company uses the Black-Scholes option pricing model to determine the estimated fair value for stock-based awards. Option-pricing models require the input of various subjective assumptions, including the option’s expected life, expected dividend yield, price volatility, risk free interest rate and forfeitures of the underlying stock. Due to the limited operating history of the Company as a public entity and a lack of company specific historical and implied volatility data, the Company has based its estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. When selecting these public companies on which it has based its expected stock price volatility, the Company selected companies with comparable characteristics to it, including enterprise value, risk profiles, position within the industry, and with historical
share price information sufficient to meet the expected term of the stock-based awards. The Company computes historical volatility data using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of the stock-based awards. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available.
Due to the lack of Company specific historical option activity, the Company has estimated the expected term of its employee stock options using the “simplified” method, whereby, the expected term equals the arithmetic average of the vesting term and the original contractual term of the option. The expected term for non-employee awards is the remaining contractual term of the option. The risk-free interest rates are based on the US Treasury securities with a maturity date commensurate with the expected term of the associated award. The Company has never paid, and does not expect to pay dividends in the foreseeable future.
All excess tax benefits and tax deficiencies are recorded as income tax expense or benefit in the Company's statement of operations and comprehensive loss. For the years ended December 31, 2018 and 2017, the Company did not record an income statement benefit for excess tax benefits as a valuation allowance is also required on these amounts.
Contingencies
Accruals are recorded for loss contingencies when it is probable that a liability has been incurred and the amount of the related loss can be reasonably estimated. The Company evaluates, on a quarterly basis, developments in legal proceedings and other matters that could cause an increase or decrease in the amount of the liability that has been accrued previously. Considering facts known at the time of the assessment, the Company determines whether potential losses are considered reasonably possible or probable and whether they are estimable. Based upon this assessment, the Company carries out an evaluation of disclosure requirements and considers possible accruals in the financial statements.
Segment Reporting
Operating segments are identified as components of an enterprise where separate discrete financial information is available for evaluation by the chief operating decision maker in making decisions on how to allocate resources and assess performance. The Company operates as a single segment dedicated to the discovery and development of biotechnological applications and the Company’s chief operating decision maker, or CODM, makes decisions based on the Company as a whole. The Company has determined that its CODM is its Chief Executive Officer.
Earnings per Share
Basic earnings per share attributable to common stockholders is calculated by dividing net loss attributable to common stockholders by the weighted average shares outstanding during the period, without consideration for common stock equivalents.
Diluted earnings per share attributable to common stockholders is calculated by adjusting weighted average shares outstanding for the dilutive effect of common stock equivalents outstanding for the period, determined using the treasury-stock and if-converted methods. For purposes of the diluted net loss per share attributable to common stockholders' calculation, preferred stock, stock options, unvested restricted stock, and warrants are considered to be common stock equivalents but have been excluded from the calculation of diluted net loss per share attributable to common stockholders, as their effect would be anti-dilutive for all periods presented. Therefore, basic and diluted net loss per share were the same for all periods presented.
Recent Accounting Pronouncements
Standards not yet adopted
In May 2014, the FASB issued Accounting Standard Update, or ASU, No. 2014-09, Revenue from Contracts with Customers (Topic 606), or ASU 2014-09. Subsequently, the FASB also issued ASU 2015-14, Revenue from Contracts with Customers (Topic 606) , which adjusted the effective date of ASU 2014-09; ASU No. 2016-08, Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations (Reporting Revenue Gross versus Net), which amends the principal-versus-agent implementation guidance and illustrations in ASU 2014-09; ASU No. 2016-10, Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing, which clarifies identifying performance obligation and licensing implementation guidance and illustrations in ASU 2014-09; and ASU No. 2016-12, Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients, which addresses implementation issues and is intended to reduce the cost and complexity of applying the new revenue standard in ASU 2014-09, collectively referred to as the Revenue ASUs, or ASC 606.
The Revenue ASUs provide an accounting standard for a single comprehensive model for use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance. The accounting standard is effective for public emerging growth companies, like the Company, for interim and annual periods beginning after December 15, 2018, with an option to early adopt for interim and annual periods beginning after December 15, 2017. The guidance permits two methods of adoption: retrospectively to each prior reporting period presented (the full retrospective method), or retrospectively with the cumulative effect of initially applying the guidance recognized at the date of initial application (the modified retrospective method). The Company plans to use the modified retrospective method with an effective date of adoption of January 1, 2019. The Company is still evaluating the potential impact that these ASUs will have on the financial position and results of operations and expects the adoption of this guidance to have a potential material impact on its consolidated financial statements stemming from the allocation of the transaction price to performance obligations, the related timing of revenue recognition for the identified performance obligations, and the impact of incremental direct costs incurred related to the acquisition or origination of customer contracts.
In February 2016, the FASB issued ASU No. 2016-2, Leases (Topic 842), or ASU 2016-2. Under the amendments in ASU 2016-2, lessees will be required to recognize (i) a lease liability, which is a lessees obligation to make lease payments arising from a lease, measured on a discounted basis; and (ii) a right-of-use asset, which is an asset that represents the lessee’s right to use, or control the use of, a specified asset for the lease term for all leases (with the exception of short-term leases) at the commencement date. This guidance is effective for public emerging growth companies, like the Company, for fiscal years beginning after December 15, 2019 including interim periods within those fiscal years. Early adoption is permitted. The Company anticipates an effective date of adoption for this standard in the fourth quarter of 2019, when the Company anticipates losing emerging growth company status. The Company has begun to assess the current state of accounting for leases, to catalog all current leases effected and understand the gaps between the current state and required future state and to implement the new processes and controls required. The Company currently expects that adoption of this standard will increase both total assets and liabilities in its consolidated financial statements and is currently evaluating whether the impact will be material.
In June 2018, the FASB issued ASU No. 2018-07, Compensation - Stock Compensation (Topic 718) Improvements to Nonemployee Share-Based Payment Accounting, or ASU 2018-07. ASU 2018-07 expands the scope of Topic 718 to include share-based payment transactions when acquiring goods and services from nonemployees as part of the ongoing operations of the business. ASU 2018-07 will not apply in financing or revenue-based transactions. Upon adoption of ASU 2018-07, the requirements of Topic 718 will apply such that the Company must establish the value of nonemployee awards at the date of grant, rather than remeasure the value over the life of the award. ASU 2018-07 does not change either the inputs required when pricing the option or the attribution of cost (the vesting pattern and pattern of cost recognition over that period). This guidance is effective for fiscal years beginning after December 15, 2018, including interim periods within that fiscal year. As Topic 606 has not yet been adopted, early adoption of ASU 2018-07 is not permissible. The Company expects to adopt this standard as of January 1, 2019 in conjunction with adoption of ASC 606 and does not expect such adoption will have a material impact on its financial statements and related disclosures given the limited number of awards to non-employees.
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework – Changes to the Disclosure Requirements for Fair Value Measurement, or ASU 2018-13. ASU 2018-13 modifies the disclosure requirements on fair value measurements by removing the requirement to disclose: the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy; the policy for timing of transfers between levels; and the valuation processes for Level 3 fair value measurements. ASU 2018-13 also requires disclosure of changes in unrealized gains and losses for the period included in other comprehensive income (loss) for recurring Level 3 fair value measurements held at the end of the reporting period and the range and weighted average of significant unobservable inputs used to develop Level 3 fair value
measurements. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. The Company does not have any Level 3 fair value instruments and thus does not expect a material impact from the adoption of ASU 2018-13.
In November 2018, the FASB issued ASU No. 2018-18, Collaborative Arrangements (Topic 808):Clarifying the Interaction between Topic 808 and Topic 606, or ASU 2018-18. ASU 2018-13 makes targeted improvements to generally accepted accounting principles for collaborative arrangements, including: clarification that certain transactions between collaborative arrangement participants should be accounted for as revenue under ASC 606 when the collaborative arrangement participant is a customer in the context of a unit of account; adding unit-of-account guidance in Topic 808 to align with the guidance in ASC 606; and a requirement that in a transaction with a collaborative arrangement participant that is not directly related to sales to third parties, presenting the transaction together with revenue recognized under ASC 606 is precluded if the collaborative arrangement participant is not a customer. This guidance is effective for fiscal years beginning after December 15, 2019, including interim periods within that fiscal year. The Company is currently evaluating the impact of adoption, if any, along with the ability for early adoption, if possible, that this standard may have on its consolidated financial statements.
The Company has considered other recent accounting pronouncements and concluded that they are either not applicable to the business, or that the effect is not expected to be material to the unaudited condensed consolidated financial statements as a result of future adoption.
3. Revenue
General
The Company has not generated revenue from product sales. The Company has generated revenue pursuant to (i) license and collaboration agreements, which include upfront payments for licenses or options to obtain licenses, payments for research and development services and milestone payments, and (ii) government grants. During the years ended December 31, 2018 and 2017, respectively, the Company recognized revenues as follows (in thousands):
|
| | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 |
License fees | $ | 26,677 |
| | $ | 11,285 |
|
Research and development services | 1,762 |
| | 1,403 |
|
Milestone payments | 571 |
| | 12,573 |
|
Other revenues | 91 |
| | 14 |
|
Total Revenue | $ | 29,101 |
| | $ | 25,275 |
|
During the years ended December 31, 2018 and 2017, respectively, the Company recognized revenue from the following strategic partnerships and other license agreements (in thousands):
|
| | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 |
AstraZeneca | $ | 17,632 |
| | $ | 19,769 |
|
Seattle Genetics | 5,413 |
| | — |
|
Servier | 4,508 |
| | 1,907 |
|
Other | 1,548 |
| | 3,599 |
|
Total Revenue | $ | 29,101 |
| | $ | 25,275 |
|
Under the Company´s existing strategic partnerships and other license agreements, the Company could receive the following potential milestone payments (in millions):
|
| | | | | | | |
| Research, Development, Regulatory & Commercial Milestones | | Sales Milestones |
AstraZeneca | $ | 1,111 |
| | $ | 960 |
|
Servier | 992 |
| | 899 |
|
Seattle Genetics | 769 |
| | 450 |
|
Total potential milestone payments | $ | 2,872 |
| | $ | 2,309 |
|
Strategic Partnerships
Seattle Genetics
On February 8, 2018, the Company entered into the Seattle Genetics Agreements with Seattle Genetics, pursuant to which the parties will develop multiple targeted bispecific IO treatments for solid tumors and blood cancers.
Under the terms of the Seattle Genetics Agreements, the companies will pursue multiple antibody-Anticalin fusion proteins during the research phase. The Seattle Genetics Agreements provides Seattle Genetics a base option to select up to three programs for further development. Prior to the initiation of a pivotal trial, the Company may opt into global co-development and US commercialization of the second program and share in global costs and profits on an equal basis. Seattle Genetics will solely develop, fund and commercialize the other two programs. Seattle Genetics may also decide to select additional candidates from the initial research phase for further development in return for the payment to us of additional fees, milestone payments, and royalties
The Seattle Genetics Platform License grants Seattle Genetics a non-exclusive license to certain intellectual property related to the Anticalin platform technology.
Upon signing the Seattle Genetics Agreements, Seattle Genetics paid the Company a $30.0 million upfront fee and an additional $4.9 million was estimated to be paid for research and development services as reimbursement to the Company through the end of the research term. In addition, the Company may receive tiered royalties on net sales up to the low double-digits and up to $1.2 billion in total success-based research, development, commercial, and sales milestones payments across the product candidates, depending on the successful development and commercialization of those candidates. If Seattle Genetics exercises its option to select additional candidates from the initial research phase for further development, payment to Pieris of additional fees, milestone payments, and royalties would result.
The term of each of the Seattle Genetics Agreements ends upon the expiration of all of Seattle Genetics’s payment obligations under such Seattle Genetics Agreement. The Seattle Genetics Collaboration Agreement may be terminated by Seattle Genetics on a product-by-product basis for convenience beginning 12 months after its effective date upon 90 days' notice or, for any program where a pivotal study has been initiated, upon 180 days' notice. Any program may be terminated at Seattle Genetics's option. If any program is terminated by Seattle Genetics after a pre-defined pre-clinical stage, the Company will have full rights to continue such program. If any program is terminated by Seattle Genetics prior to such pre-defined pre-clinical stage, the Company will have the right to continue to develop such program, but will be obligated to offer a co-development option to Seattle Genetics for such program. The Seattle Genetics Collaboration Agreement may also be terminated by Seattle Genetics or the Company for an uncured material breach by the other party upon 90 days' notice, subject to extension for an additional 90 days if the material breach relates to diligence obligations and subject, in all cases, to dispute resolution procedures. The Seattle Genetics Collaboration Agreement may also be terminated due to the other party’s insolvency and may in certain instances, including for reasons of safety, be terminated on a product-by-product basis. Each party may also terminate the Seattle Genetics Agreements if the other party challenges the validity of any patents licensed under the Seattle Genetics Agreements, subject to certain exceptions. The Seattle Genetics Platform License will terminate upon termination of the Seattle Genetics Collaboration Agreement, whether in its entirety or on a product-by-product basis.
The Company accounted for the Seattle Genetics Agreements as a multiple element arrangement under ASC 605-25. The arrangement with Seattle Genetics contains the following initial deliverables: (i) three candidate research licenses that each consist of a non-exclusive platform technology license, a co-exclusive candidate research license, and research and development services, (ii) research, development and manufacturing services associated with each candidate research license, (iii) participation on various governance committees, and (iv) two antibody target swap options.
Management considered whether any of the deliverables could be considered separate units of accounting. The Company determined each license granted, at arrangement inception, did not have standalone value from the research and development services to be provided for the related antibody target programs due to the specific nature of the intellectual property and knowledge required to perform the research and development services. The Company determined that the participation on the
various governance committees did have standalone value from the delivered licenses as the services could be performed by an outside party. The Company determined that the two antibody target swap options did have stand-alone value from the delivered licenses as the absence of delivering swap options does not impact the delivery of either the research licenses or the research, development and manufacturing services associated with each candidate research license.
As a result, management concluded there are six units of accounting at inception of the Seattle Genetics Agreements: (i) three combined units of accounting each representing a non-exclusive platform technology license, a co-exclusive candidate research license, and research and development services for first three approved Seattle Genetics antibody target programs, (ii) two units of accounting each representing an antibody target swap right for first and the second approved Seattle Genetics antibody target, and (iii) one unit of accounting representing the participation of the various governance committees.
The Company determined that neither VSOE nor TPE is available for any of the units of accounting identified at arrangement inception. Accordingly, the selling price of each unit of accounting was developed using BESP. The Company developed its best estimate of selling price for licenses by applying a risk adjusted, net present value, estimate of future potential cash flows approach, which included the cost of obtaining research and development services at arm’s length from a third-party provider, as well as internal full-time equivalent costs to support these services.
The Company developed the BESP for committee participation by using management’s best estimate of the anticipated participation hours multiplied by a market rate for comparable participants.
Allocable arrangement consideration at inception was comprised of the upfront fees of $30.0 million and $4.9 million of estimated research and development services to be reimbursed as research and development occurs through the research term. Therefore, the total allocable arrangement consideration at inception was $34.9 million and was allocated among the separate units of accounting using the relative selling price method.
The amounts allocated to the combined units of accounting for the three research programs will be recognized on a proportional performance basis through the completion of each respective estimated research term for the individual research programs. However, for the first two programs that contain antibody target swap rights, if the antibody target swap right is exercised, any remaining deferred revenue associated with the two programs at the time of the exercise would be recognized immediately. The amounts allocated to the antibody target swap rights will be recognized either at the time the target right expires, or if exercised, on a proportional performance basis over the estimated research term for that program. The amounts allocated to the participation on each of the committees will be recognized ratably over the anticipated research term for all research programs.
Management determined that all research, development, commercial and sales milestones are deemed non-substantive as they are based solely on the performance of another party. Non-substantive milestones will be treated as contingent revenue and will be recognized when achieved, to the extent the Company has no remaining performance obligations under the arrangement. Milestone payments earned upon the achievement of sales events will be recognized when earned.
The Company will recognize royalty revenue in the period of sale for the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and the Company has no remaining performance obligations, assuming all other revenue recognition criteria are met.
As of December 31, 2018, there is $4.6 million and $18.5 million of current and non-current deferred revenue, respectively, related to the Seattle Genetics Agreements.
AstraZeneca
On May 2, 2017, the Company entered into the AstraZeneca Agreements with AstraZeneca, which became effective on June 10, 2017, following expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976. Under the AstraZeneca Agreements the parties will advance several novel inhaled Anticalin proteins.
In addition to the AstraZeneca Lead Product, the Company and AstraZeneca will also collaborate to progress four additional novel Anticalin proteins against undisclosed targets for respiratory diseases, or the AstraZeneca Collaboration Products, and together with the AstraZeneca Lead Product, collectively referred to as the AstraZeneca Products. The Company is responsible for advancing the AstraZeneca Lead Product through the phase 1 study, with the associated costs funded by AstraZeneca. The parties will collaborate thereafter to conduct a phase 2a study in asthma patients, with AstraZeneca continuing to fund development costs. After completion of the phase 2a study, Pieris has the option to co-develop the AstraZeneca Lead Product and also has the option to co-commercialize the AstraZeneca Lead Product in the United States. For the four AstraZeneca Collaboration Products, the Company will be responsible for the initial discovery of the novel Anticalin proteins, after which AstraZeneca will take the lead on continued development. The Company has the option to co-develop two of these four AstraZeneca Collaboration Products beginning at a pre-defined preclinical stage and would also have the option to co-commercialize these two programs in the United States, while AstraZeneca will be responsible for development and commercialization of the other programs worldwide.
The term of each of the AstraZeneca Agreements ends upon the expiration of all of AstraZeneca’s payment obligations under such AstraZeneca Agreement. The AstraZeneca Collaboration Agreement may be terminated by AstraZeneca in its entirety for convenience beginning 12 months after its effective date upon 90 days’ notice or, if the Company has obtained marketing approval for the marketing and sale of a product, upon 180 days’ notice. Each program may be terminated at AstraZeneca’s option; if any program is terminated by AstraZeneca, the Company will have full rights to such program. The AstraZeneca Collaboration Agreement may also be terminated by AstraZeneca or the Company for material breach upon 180 days’ notice of a material breach (or 30 days with respect to payment breach), provided that the applicable party has not cured such breach by the permitted cure period (including an additional 180 days if the breach is not susceptible to cure during the initial 180-day period) and dispute resolution procedures specified in the applicable AstraZeneca Agreement have been followed. The AstraZeneca Collaboration Agreement may also be terminated due to the other party’s insolvency and may in certain instances be terminated on a product-by-product and/or country-by-country basis. Each party may also terminate an AstraZeneca Agreement if the other party challenges the validity of patents related to certain intellectual property licensed under such AstraZeneca Agreement, subject to certain exceptions for infringement suits, acquisitions and newly-acquired licenses. The AstraZeneca Platform License will terminate upon termination of the AstraZeneca Collaboration Agreement, on a product-by-product and/or country-by-country basis.
At inception, AstraZeneca is granted the following licenses: (i) research and development license for the AstraZeneca Lead Product, (ii) commercial license for the AstraZeneca Lead Product, (iii) individual research licenses for each of the four AstraZeneca Collaboration Products, (iv) individual commercial licenses for each of the four AstraZeneca Collaboration Products, and (v) individual non-exclusive platform technology licenses for the AstraZeneca Lead Product and the four AstraZeneca Collaboration Products. AstraZeneca will be granted individual development licenses for each of the four AstraZeneca Collaboration Products upon completion of the initial discovery of Anticalin proteins.
The collaboration will be managed on an overall basis by a Joint Steering Committee, or JSC, formed by an equal number of representatives from the Company and AstraZeneca. In addition to the JSC, the AstraZeneca Collaboration Agreement also requires each party to designate an Alliance Manager to facilitate communication and coordination of the Parties activities under that AstraZeneca Agreement, as well as requires participation of both parties on: (i) a Joint Development Committee, or JDC, and (ii) a Commercialization Committee. The responsibilities of these committees vary, depending on the stage of development and commercialization of each Product.
Under the AstraZeneca Agreements, the Company received an upfront, non-refundable payment of $45.0 million. In addition, the Company will receive payments to conduct a phase 1 clinical study for the PRS-060.The Company is also eligible to receive research, development, commercial, sales milestone payments, and royalty payments. The Company may receive tiered royalties on sales of potential products commercialized by AstraZeneca and for co-developed products, gross margin share on worldwide sales equal dependent on the Company’s level of committed investment.
The Company accounted for the AstraZeneca Agreements as a multiple element arrangement under ASC 605-25. The arrangement with AstraZeneca contains the following initial deliverables: (i) five non-exclusive platform technology licenses, (ii) research and development license for the AstraZeneca Lead Product, (iii) commercial license for the AstraZeneca Lead Product, (iv) development and manufacturing services for the AstraZeneca Lead Product, (v) technology transfer services for the AstraZeneca Lead Product, (vi) research services related to the AstraZeneca Lead Product, (vii) participation on each of the committees, (viii) four research licenses for the AstraZeneca Collaboration Products, (ix) four commercial licenses for the AstraZeneca Collaboration Products, and (x) research services for the AstraZeneca Collaboration Products. Additionally, as the development licenses on the four AstraZeneca Collaboration Products may be granted at a discount in the future, the Company determined such discounts be included as an element of the arrangement at inception.
Management considered whether any of the deliverables could be considered separate units of accounting. The Company determined that the licenses granted for the AstraZeneca Lead Product at the inception of the arrangement did not have standalone value from the research services related to the AstraZeneca Lead Product and the licenses granted for the AstraZeneca Collaboration Products did not have standalone value from the research services for the AstraZeneca Collaboration Products, due to the specific nature of the intellectual property and knowledge required to perform the services. The Company also determined that the licenses granted at the inception of the arrangement did have standalone value from the development and manufacturing services for the AstraZeneca Lead Product, but that the participation on the various committees did have standalone value as the development and manufacturing services and committee service could be performed by an outside party. The Company determined that the commercial licenses for the AstraZeneca Collaboration Products granted at the inception of the arrangement did not have standalone value from the development licenses for the AstraZeneca Collaboration Products as the company would not benefit from the commercial license without the ability to develop each product.
As a result, management concluded that there were 11 units of accounting at the inception of the AstraZeneca Agreements: (i) combined unit of accounting representing a non-exclusive platform technology license, research and development license, and commercial licenses for the AstraZeneca Lead Product and research services for the AstraZeneca Lead Product, (ii) combined unit of accounting representing development and manufacturing services, and technology transfer services for the AstraZeneca Lead Product, (iii) committee participation, (iv-vii) four units of accounting representing a combined non-exclusive platform technology license, research licenses, and research services for each AstraZeneca Collaboration Product, and (viii-xi) four units of accounting representing the combined commercial licenses granted for the AstraZeneca Collaboration Products and corresponding discounts on the development licenses granted for the AstraZeneca Collaboration Products upon the achievement of specified preclinical activities.
The Company determined that neither VSOE nor TPE is available for any of the units of accounting identified at the inception of the arrangement. Accordingly, the selling price of each unit of accounting was developed using management’s BESP. The Company developed the BESP for licenses and corresponding research services by applying a risk adjusted, net present value, estimate of future potential cash flow approach, which included the cost of obtaining research services at arm’s length from a third-party provider, as well as internal full-time equivalent costs to support these services. The Company developed the BESP for development and manufacturing services, and technology transfer services for the AstraZeneca Lead Product using estimated internal and external costs to be incurred.
The Company developed the BESP for committee participation by using management’s best estimate of the anticipated participation hours multiplied by a market rate for comparable participants.
The Company developed the BESP for the commercial licenses and discounts granted on the development licenses by probability weighting multiple cash flow scenarios using the income approach.
Allocable arrangement consideration at inception is comprised of the $45.0 million upfront fee and the $8.2 million estimated development and manufacturing services to be reimbursed for the AstraZeneca Lead Product. The aggregate allocable consideration of $53.2 million is allocated among the separate units of accounting using the relative selling price method.
The amounts allocated to the unit of accounting for the AstraZeneca Lead Product and four units of accounting for the four AstraZeneca Collaboration Products will be recognized on a proportional performance basis as the activities are conducted over the life of the arrangement. The amounts allocated to the development and manufacturing services, and technology transfer services for the AstraZeneca Lead Product will be recognized on a proportional performance basis over the estimated term of development through phase 2a study. The amounts allocated to the participation on each of the committees will be recognized on a straight-line basis over the expected term of development of the AstraZeneca Lead Product and the AstraZeneca Collaboration Products. The term of performance at the inception of the arrangement is approximately five years. The amounts allocated to the commercial licenses and discounts on the development licenses granted in the future for the AstraZeneca Collaboration Products will be recognized upon delivery of each of the development licenses assuming all other revenue recognition criteria have been met. Additionally, the Company evaluated payments required to be made between both parties as a result of the shared development costs of the AstraZeneca Lead Product and the two AstraZeneca Collaboration Products for which the Company has a co-development option. The Company will classify payments made as a reduction of revenue and will classify payments received as revenue, in the period they are earned.
Under the AstraZeneca Agreements, the Company is eligible to receive various research, development, commercial, and sales milestones. Management determined certain of the research, development, and commercial milestones that may be received under the AstraZeneca Agreements are substantive when the Company is involved in the development and commercialization of the applicable AstraZeneca Products. Payment related to achievement of such milestones, if any, will be recognized as revenue when the milestone is achieved. Total potential substantive development milestones range from $72.2 million to $611.4 million, dependent on the Company’s decision, on a product-by-product basis, whether to co-develop the AstraZeneca Lead Product and AstraZeneca Collaboration Products. Research, development, and commercial, and sales milestones are deemed non-substantive if they are based solely on the performance of another party. Non-substantive milestones will be treated as contingent revenue and will be recognized when achieved to the extent the Company has no remaining performance obligations under the arrangement. Total potential non-substantive research, development, and commercial milestones range from $366.2 million to $1.1 billion. The Company may receive lower research, development, and commercial, milestones if the Company chooses to co-develop the AstraZeneca Lead Product and/or AstraZeneca Collaboration Products, depending on the level of co-development investment. Total potential sales milestones are up to $1.0 billion and will be recognized when earned, assuming all other revenue recognition revenue criteria have been met.
The Company will recognize royalty and gross margin share revenue in the period of sale of the related AstraZeneca Product, based on the underlying contract terms, provided that the reported sales are reliably measurable and the Company has no remaining performance obligations, assuming all other revenue recognition criteria are met.
The successful initiation of a phase 1 study during the year ended December 31, 2017 resulted in a milestone achievement of $12.6 million, which was recorded as revenue in the respective period. We are eligible to receive another milestone payment for PRS-060 upon the initiation of a phase 2a study. As of December 31, 2018, there is $25.5 million and $6.8 million of current and non-current deferred revenue, respectively, related to the AstraZeneca Agreements.
Servier
On January 4, 2017, the Company entered into the Servier Agreements with Servier pursuant to which the Company and Servier will initially pursue five bispecific therapeutic programs.
Five committed programs have been defined, which may combine antibodies from the Servier portfolio with one or more Anticalin proteins based on the Company’s proprietary platform to generate innovative IO bispecific drug candidates, or the Collaboration Products. The collaboration may be expanded by up to three additional therapeutic programs. The Company has the option to co-develop and retain commercial rights in the United States for up to three programs beyond PRS-332, or the Co-Development Collaboration Products, while Servier will be responsible for development and commercialization of the other programs worldwide, or the Servier Worldwide Collaboration Products. Each party is responsible for an agreed upon percentage of shared costs, as set forth in the budget for the collaboration plan, and further discussed below.
Co-Development Collaboration Products may be jointly developed, according to a collaboration plan, through marketing approval from the FDA or EMA. Servier Worldwide Collaboration Products may be jointly developed, according to a collaboration plan, through specified preclinical activities, at which point Servier becomes responsible for further development of the Collaboration Product.
At inception, Servier was granted the following licenses: (i) development license for PRS-332, (ii) commercial license for PRS-332, (iii) individual research licenses for each of the four Collaboration Products, and (iv) individual non-exclusive platform technology licenses for each of PRS-332 and four Collaboration Products. Upon achievement of certain development activities, specified by the collaboration for each Servier Agreement, Servier will be granted a development license and a commercial license. For PRS-332 and Co-Development Collaboration Products, the licenses granted are with respect to the entire world except for the United States. For Servier Worldwide Collaboration Products, the licenses granted are with respect to the entire world.
The Servier Agreements will be managed on an overall basis by a joint executive committee, or JEC, formed by an equal number of members from the Company and Servier. Decisions by the JEC will be made by consensus, however, in the event of a disagreement, each party will have final-decision making authority as it relates to the applicable territory in which such party has commercialization rights for the applicable product. In addition to the JEC, the Servier Collaboration Agreement requires the participation of both parties on: (i) a JSC, (ii) a JDC, (iii) a Joint Intellectual Property Committee, or JIPC, and (iv) a Joint Research Committee, or JRC. The responsibilities of these committees vary, depending on the stage of development and commercialization of PRS-332 and each of the Collaboration Products.
For PRS-332 and Co-Development Collaboration Products, the Company and Servier are responsible for an agreed upon percent of the shared costs required to develop the products through commercialization. In the event that the Company fails to exercise their option to co-develop the Co-Development Collaboration Products, Servier has the right to continue with the development and will be responsible for all costs required to develop the products through commercialization.
Under the Servier Agreements, the Company received an upfront, non-refundable payment of €30.0 million (approximately $32.0 million). In addition, the Company is eligible to receive research, development, commercial, and sales milestone payments as well as tiered royalties up to low double digits on the sales of commercialized products in the Servier territories. The Company achieved two preclinical milestones under the program, one in December 2018 for €0.5 million (approximately $0.6 million) and another in February 2019 for €1.5 million (approximately $1.7 million), both of which were paid in 2019. The Company intends to file an IND for the drug candidate in the second half of 2019 which would trigger another milestone payment due from Servier.
The initial research collaboration term, as it relates to PRS-332 and Collaboration Products, shall continue for three years from the effective date, and may be mutually extended for two one-year terms consecutively applied.
The term of each Servier Agreement ends upon the expiration of all of Servier’s payment obligations under such Servier Agreement. The Servier Agreements may be terminated by Servier for convenience beginning 12 months after their effective date upon 180 days’ notice. The Servier Agreements may also be terminated by Servier or the Company for material breach upon 90 days’ or 120 days’ notice of a material breach, with respect to the Servier Collaboration Agreement and the Servier Platform License, respectively, provided that the applicable party has not cured such breach by the applicable 90-day or 120-day permitted cure period, and dispute resolution procedures specified in the applicable Servier Agreement have been followed. The Servier Agreements may also be terminated due to the other party’s insolvency or for a safety issue and may in certain instances be terminated on a product-by-product and/or country-by-country basis. The Servier Platform License will terminate upon termination of the Servier Collaboration Agreement, on a product-by-product and/or country-by-country basis.
The Company accounted for the Servier Agreements as a multiple element arrangement under ASC 605-25. The arrangement with Servier contains the following initial deliverables: (i) five non-exclusive platform technology licenses, (ii) development license for PRS-332, (iii) commercial license for PRS-332, (iv) research and development services for PRS-332, (v) participation on each of the committees, (vi) four research licenses for Collaboration Products, and (vii) research and development services for the Collaboration Products. Additionally, as the development and commercial licenses on the four Collaboration Products may be granted at discount in the future, the Company determined such discounts be included as an element of the arrangement at inception.
Management considered whether any of the deliverables could be considered separate units of accounting. The Company determined the licenses granted, at arrangement inception, did not have standalone value from the research and development services to be provided for PRS-332 and Collaboration Products, over the term of the Servier Agreements, due to the specific nature of the intellectual property and knowledge required to perform the research and development services. The Company determined that the participation on the various committees did have standalone value from the delivered licenses as the services could be performed by an outside party.
As a result, management concluded there are 10 units of accounting at inception of the Servier Agreements: (i) combined unit of accounting representing a non-exclusive platform technology license, commercial license, development license and research and development services for PRS-332, (ii) four units of accounting each representing a combined non-exclusive platform technology license, research license, and research and development services for each Collaboration Product (iii) one unit of accounting representing the participation of the various governance committees, and (iv) four units of accounting representing the discounts on the development and commercial licenses granted for the Collaboration Products upon the achievement of specified preclinical activities.
The Company determined that neither VSOE nor TPE is available for any of the units of accounting identified at arrangement inception. Accordingly, the selling price of each unit of accounting was developed using BESP. The Company developed its best estimate of selling price for licenses by applying a risk adjusted, net present value, estimate of future potential cash flows approach, which included the cost of obtaining research and development services at arm’s length from a third-party provider, as well as internal full-time equivalent costs to support these services.
The Company developed the BESP for committee participation by using management’s best estimate of the anticipated participation hours multiplied by a market rate for comparable participants.
The Company developed the BESP for the discounts granted on the licenses by probability weighting multiple cash flow scenarios using the income approach.
Allocable arrangement consideration at inception is comprised of the upfront fee of €30.0 million (approximately $32.0 million) and was allocated among the separate units of accounting using the relative selling price method.
The amounts allocated to the combined unit of accounting for PRS-332 and four units of accounting for the four Collaboration Products will be recognized on a proportional performance basis as the activities are conducted over the life of the arrangement. The term of the performance at inception of the Servier Agreements for PRS-332 and each of the Co-Developed Collaboration Products may be through approval of certain regulatory bodies; a period which could be many years. The term of the performance at inception of the agreement for each of the other two Servier Worldwide Collaboration Products is approximately two to three years. The amounts allocated to the participation on each of the committees will be recognized ratably over the anticipated performance period over the entirety of the arrangement with Servier. The amounts allocated to the
discounts of the development and commercial licenses granted in the future will be recognized upon delivery of each of the licenses assuming no other performance obligations.
Additionally, the Company evaluated payments required to be made between both parties as a result of the shared development costs of PRS-332 and Co-Development Collaboration Products. The Company will classify payments made as a reduction of revenue and will classify payments received as revenue, in the period they are earned.
Under the Servier Agreements the Company is eligible to receive various research, development, commercial, and sales milestones. Management determined certain research, development and commercial milestones, which may be received under the Servier Agreements, are substantive when the Company is involved in the development and commercialization of the applicable product. Payments related to the achievement of such milestones, if any, will be recognized as revenue when the milestone is achieved. Total potential substantive research, development and commercial milestones are up to €163.0 million. Research, development, and commercial milestones are deemed non-substantive if they are based solely on the performance of another party. Non-substantive milestones will be treated as contingent revenue and will be recognized when achieved, to the extent the Company has no remaining performance obligations under the arrangement. Milestone payments earned upon the achievement of sales events will be recognized when earned.
The Company will recognize royalty revenue in the period of sale for the related product(s), based on the underlying contract terms, provided that the reported sales are reliably measurable and the Company has no remaining performance obligations, assuming all other revenue recognition criteria are met.
As of December 31, 2018, there is $2.6 million and $28.0 million of current and non-current deferred revenue, respectively, related to the Servier Agreements.
Other License Agreements
ASKA
On February 27, 2017 the Company entered into the ASKA Option Agreement to grant ASKA an option to acquire (1) a non-exclusive license to certain intellectual property rights associated with the Company’s Anticalin platform, or the Licensed Platform IP, and (2) an exclusive license to certain intellectual property rights specifically related to the Company’s PRS-080 Anticalin protein, or the Licensed Product IP, in order to develop, manufacture, import, sale, export, and offer for sale and export any pharmaceutical formulation containing PRS-080, the Company’s PEGylated Anticalin protein targeting hepcidin, or the Licensed Product, in Japan and certain other Asian territories, or the Licensed Territory.
Pieris is obliged to use commercially reasonable efforts to complete the phase 2a study for PRS-080 and to submit to ASKA, in writing, the final results of the study when available. Upon receipt, ASKA will have 60 days to evaluate the results of the phase 2a study, or the Evaluation Period. ASKA agreed to notify the Company, in writing, of its decision to exercise its option to acquire rights to the Licensed Product. If the phase 2a study meets the applicable success criteria and ASKA fails to provide notification that it will exercise its option, ASKA shall pay the Company an additional fee within 30 days of the end of the Evaluation Period, or the Break-Up Fee. If ASKA exercises the option, ASKA and the Company will enter into a separate definitive arrangement governing the future development and commercialization activities.
The Company determined that the completed phase 2a study represents the single deliverable (and the sole unit of account) under the ASKA Option Agreement for which an upfront payment of $2.75 million was received from ASKA. While the completion of the phase 2a study requires the completion of a number of actions, the Company determined that the significance in value of the finalization of the data and evaluation of results of the phase 2a study is the point at which revenue would be recognized. Therefore, no revenue was recognized in connection with this arrangement for the years ended December 31, 2018 and 2017, respectively. As of December 31, 2018, there is $2.94 million of current deferred revenue related to the ASKA Option Agreement.
4. Cash, Cash Equivalents and Investments
As of December 31, 2018 and 2017, cash, cash equivalents and investment comprised funds in depository, money market accounts, US treasury securities, asset backed securities, and corporate bonds. The following table presents the cash equivalents and investments carried at fair value in accordance with the hierarchy defined in Note 2 at December 31, 2018 and 2017 (in thousands):
|
| | | | | | | | | | | | |
| Total | Quoted prices in active markets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) |
December 31, 2018 | | | | |
Money market funds, included in cash equivalents | $ | 7,791 |
| $ | 7,791 |
| $ | — |
| $ | — |
|
Corporate bonds, included in cash equivalents | 10,910 |
| — |
| 10,910 |
| — |
|
Investments - US treasuries | 7,518 |
| 7,518 |
| — |
| — |
|
Investments - Asset-backed securities | 5,758 |
| — |
| 5,758 |
| — |
|
Investments - Corporate bonds | 39,964 |
| — |
| 39,964 |
| — |
|
Total | $ | 71,941 |
| $ | 15,309 |
| $ | 56,632 |
| $ | — |
|
|
| | | | | | | | | | | | |
| Total | Quoted prices in active markets (Level 1) | Significant other observable inputs (Level 2) | Significant unobservable inputs (Level 3) |
December 31, 2017 | | | | |
Money market funds, included in cash equivalents | $ | 4,583 |
| $ | 4,583 |
| $ | — |
| $ | — |
|
Corporate bonds, included in cash equivalents | 13,595 |
| — |
| 13,595 |
| — |
|
Investments - US treasuries | 4,172 |
| 4,172 |
| — |
| — |
|
Investments - Asset-backed securities | 6,384 |
| — |
| 6,384 |
| — |
|
Investments - Corporate bonds | 34,117 |
| — |
| 34,117 |
| — |
|
Total | $ | 62,851 |
| $ | 8,755 |
| $ | 54,096 |
| $ | — |
|
Cash equivalents and marketable securities have been initially valued at the transaction price and subsequently valued, at the end of each reporting period, utilizing third party pricing services or other market observable data. The pricing services utilize industry standard valuation models, including both income and market-based approaches and observable market inputs to determine value. The Company validates the prices provided by its third-party pricing services by reviewing their pricing methods and obtaining market values from other pricing sources, as needed. After completing its validation procedures, the Company did not adjust any fair value measurements provided by the pricing services as of December 31, 2018.
Investments at December 31, 2018 consist of the following (in thousands):
|
| | | | | | | | | | | | | |
| Contractual maturity
(in days) | Amortized Cost | Unrealized gains | Unrealized losses | Fair Value |
Investments | | | | | |
US treasuries | 150-164 | $ | 7,541 |
| $ | — |
| $ | (23 | ) | $ | 7,518 |
|
Asset-backed securities | 196-259 | 5,766 |
| 1 |
| (9 | ) | 5,758 |
|
Corporate bonds | 73-252 | 40,072 |
| 3 |
| (111 | ) | 39,964 |
|
Total | | $ | 53,379 |
| $ | 4 |
| $ | (143 | ) | $ | 53,240 |
|
Investments at December 31, 2017 consist of the following (in thousands):
|
| | | | | | | | | | | | | |
| Contractual maturity
(in days) | Amortized Cost | Unrealized gains | Unrealized losses | Fair Value |
Investments | | | | | |
US treasuries | 212-340 | $ | 4,287 |
| $ | — |
| $ | (115 | ) | $ | 4,172 |
|
Asset-backed securities | 197-365 | 2,709 |
| — |
| (14 | ) | 2,695 |
|
Asset-backed securities | greater than 365 | 3,798 |
| — |
| (110 | ) | 3,688 |
|
Corporate bonds | 91-365 | 28,356 |
| — |
| (473 | ) | 27,882 |
|
Corporate bonds | greater than 365 | 6,344 |
| 1 |
| (108 | ) | 6,236 |
|
Total | | $ | 45,494 |
| $ | 1 |
| $ | (820 | ) | $ | 44,673 |
|
There were $1.0 million of realized gains for the year ended December 31, 2018. No realized gains or losses were recorded for the year ended December 31, 2017.
5. Property and Equipment, Net
Property and equipment are summarized as follows (in thousands):
|
| | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 |
Laboratory equipment | $ | 7,431 |
| | $ | 6,101 |
|
Office and computer equipment | 661 |
| | 494 |
|
Leasehold improvements | 323 |
| | 318 |
|
Property and equipment at cost | 8,415 |
| | 6,913 |
|
Accumulated depreciation | (3,366 | ) | | (2,879 | ) |
Property and equipment, net | $ | 5,049 |
| | $ | 4,034 |
|
Depreciation expense was $0.6 million and $0.4 million for the years ended December 31, 2018 and 2017, respectively. There were no other changes in accumulated depreciation other than the foreign currency impact.
6. Accrued Expenses
Accrued expenses and other current liabilities consisted of the following (in thousands):
|
| | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 |
Accrued license obligations | $ | 2,523 |
| | $ | 806 |
|
Compensation expense | 2,380 |
| | 2,325 |
|
Research and development fees | 1,945 |
| | 791 |
|
Professional fees | 943 |
| | 516 |
|
Audit and tax fees | 378 |
| | 424 |
|
Other current liabilities | 945 |
| | 1,308 |
|
Total | $ | 9,114 |
| | $ | 6,170 |
|
7. Income Taxes
The Company reported a loss before income taxes consisting of the following (in thousands):
|
| | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 |
Domestic | $ | (10,633 | ) | | $ | (13,840 | ) |
Foreign | (16,433 | ) | | (2,704 | ) |
Loss before income taxes | $ | (27,066 | ) | | $ | (16,544 | ) |
The components of the (benefit) provision for income taxes are as follows (in thousands):
|
| | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 |
Current: | | | |
Federal | $ | — |
| | $ | — |
|
State | — |
| | — |
|
Foreign | (148 | ) | | 1,103 |
|
Total current | (148 | ) | | 1,103 |
|
Deferred: | | | |
Federal | — |
| | — |
|
State | — |
| | — |
|
Foreign | (164 | ) | | — |
|
Total deferred | (164 | ) | | — |
|
(Benefit) provision for income taxes | $ | (312 | ) | | $ | 1,103 |
|
The reconciliation of the federal statutory rate to the Company’s effective tax rate is as follows:
|
| | | | | |
| 2018 | | 2017 |
Federal income tax rate | 21.0 | % | | 34.0 | % |
Tax Reform - Change in enacted rate | — |
| | (22.2 | ) |
Foreign rate differential | 7.4 |
| | (1.2 | ) |
State tax, net of federal benefit | 0.7 |
| | 2.4 |
|
US tax on foreign income | (8.1 | ) | | — |
|
Share-based awards compensation | 2.0 |
| | — |
|
Permanent items | 0.8 |
| | — |
|
Other | 0.5 |
| | 1.8 |
|
Change in valuation allowance | (23.1 | ) | | (21.5 | ) |
Effective income tax rate | 1.2 | % | | (6.7 | )% |
The components of deferred tax assets and liabilities related to net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income taxes purposes were as follows (in thousands):
|
| | | | | | | |
| Years Ended December 31, |
| 2018 | | 2017 |
Deferred tax assets: | | | |
Net operating loss carryforwards | $ | 27,879 |
| | $ | 22,170 |
|
Share-based awards compensation | 2,359 |
| | 1,280 |
|
Accrued compensation | 304 |
|
| 350 |
|
Depreciation and other | 125 |
| | 89 |
|
Deferred Revenue | 641 |
| | 1,504 |
|
Total deferred tax assets | 31,308 |
| | 25,393 |
|
Deferred tax liabilities: | | | |
Unrealized gain on investments | (394 | ) | | (143 | ) |
Other | (98 | ) | | — |
|
Total deferred tax liabilities | (492 | ) | | (143 | ) |
Less: valuation allowance: | (30,816 | ) | | (25,250 | ) |
Net deferred tax asset | $ | — |
| | $ | — |
|
The Company operates in multiple jurisdictions. Accordingly, the Company files US federal and state income tax returns as well as returns in multiple foreign jurisdictions. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax-planning strategies in making this assessment. Management believes it is more likely than not that the results of future operations will not generate sufficient taxable income in the United States or in its foreign jurisdictions to realize the full benefits of its deferred tax assets. As of December 31, 2018, we continue to maintain a full valuation allowance against all net deferred tax assets.
The cumulative amount of earnings of our foreign subsidiaries are expected to be permanently invested in the foreign subsidiaries. Deferred taxes have not been provided on the excess of book basis over tax basis, or the excess tax basis over book basis in the shares of our foreign subsidiaries because these basis differences are not expected to reverse in the foreseeable future and are essentially permanent in duration. Our intention is to reinvest the earnings of the foreign subsidiaries indefinitely.
The increase in the valuation allowance of deferred tax assets of $5.6 million was primarily influenced by the operating losses generated in current tax year.
As of December 31, 2018, the Company had net operating loss carryforwards for US federal income tax purposes of $15.3 million and net operating loss carryforwards for state income tax purposes of $20.1 million. Tax loss carryforwards that were created prior to December 31, 2017 expire through 2037, all tax loss carryforwards created after that date do not expire. In the United States, utilization of the NOL carryforwards may be subject to a substantial annual limitation under Section 382 of the Code and similar
state provisions due to ownership change limitations that have occurred previously or that could occur in the future. These ownership changes may limit the amount of NOL carryforwards that can be utilized annually to offset future taxable income and tax, respectively. The Company has not currently completed a study to assess whether an ownership change has occurred, or whether there have been multiple ownership changes since the acquisition of the US entity in 2014. Since the Company has incurred net operating losses since inception, it has never been subject to a revenue agent review. The Company is currently open to examination under the statute of limitations by the Internal Revenue Service and state jurisdictions for the tax years ended 2014 through 2018. Carryforward tax attributes generated in years past may still be adjusted upon future examination if they have or will be used in a future period. The Company is currently not under examination by the Internal Revenue Service or any other jurisdictions for any tax years.
As of December 31, 2018, the Company had German corporate income tax and trade tax net operating loss carryforwards of approximately $80.3 million and $79.4 million respectively. Under current German laws, tax loss carryforwards may only be used to offset any relevant later assessment period (calendar year) $1.2 million plus 60% of the exceeding taxable income and trade profit of such period. In addition, certain transactions, including transfers of shares or interest in the loss holding entity, may result in the partial or total forfeiture of tax losses existing at that date. Partial or total forfeiture of tax losses may further occur in corporate reorganizations of the loss holding entity. Tax years ended December 31, 2014 or later remain subject to examination by the German tax authorities.
The Company accounts for uncertain tax positions pursuant to ASC 740 which prescribes a recognition threshold and measurement process for financial statement recognition of uncertain tax positions taken or expected to be taken in a tax return. If the tax position meets this threshold, the benefit to be recognized is measured at the largest amount of benefit that is more likely than not (determined by cumulative probability) of being realized upon ultimate settlement with the taxing authority. The Company recorded an uncertain tax position related to a prior year position, that if successfully challenged by tax authorities could result in the loss of certain tax attributes. The balance of uncertain tax positions will remain until such time that settlement is reached with the relevant tax authorities or should the statute of limitations expire. The Company recognizes interest and penalties, if any, related to uncertain tax positions in income tax expense. No interest and penalties related to uncertain tax positions were accrued at December 31, 2018 and December 31, 2017.
The following table sets forth a reconciliation of the beginning and ending amounts of unrecognized tax benefits, excluding the impact of interest and penalties, for the years ended December 31, 2018 and 2017 (in thousands):
|
| | | |
Unrecognized tax benefits at December 31, 2017 | $ | 6,451 |
|
Currency translation adjustment | (294 | ) |
Unrecognized tax benefits at December 31, 2018 | $ | 6,157 |
|
The Company does not expect unrecognized tax benefits to change significantly over the next 12 months. The full amount of unrecognized tax benefits would impact the effective rate, subject to valuation allowance considerations, if recognized.
Enacted Tax Legislation
On December 22, 2017, the TCJA was signed into law making significant changes to the Internal Revenue Code. Changes include, but are not limited to, a corporate tax rate decrease from 35% to 21% effective for tax years beginning after December 31, 2017, the transition of U.S international taxation from a worldwide tax system to a territorial system, and a one-time transition tax on the mandatory deemed repatriation of cumulative foreign earnings as of December 31, 2017. ASC 740 requires the tax effects of changes in tax laws must be recognized in the period in which the law is enacted, or December 22, 2017 for the Act. ASC 740 also requires deferred tax assets and liabilities to be measured at the enacted tax rate expected to apply when temporary differences are to be realized or settled. Thus, at the date of enactment, the Company’s deferred taxes were re-measured utilizing the new federal income tax rate of 21%. On December 22, 2017, the SEC staff issued Staff Accounting Bulletin No. 118, Income Tax Accounting Implications of the TCJA, or SAB 118, which allows the recording of provisional amounts during a measurement period not to extend beyond one year of the enactment date. In accordance with SAB 118, we determined that our deferred tax asset value and associated valuation allowance reduction of $3.7 million was a provisional amount and a reasonable estimate at December 31, 2017. The Company finalized its accounting for the impact of changes in US tax laws in the three months ended December 31, 2018. No significant adjustments to the provisional amount were made.
The TCJA also subjects a US shareholder to tax on global-intangible low tax income (GILTI) earned by certain foreign subsidiaries. An entity can make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as GILTI in future years or to provide for the tax expense related to GILTI in the year the tax is incurred as a period expense only. The Company will account for GILTI in the year the tax is incurred as a period cost.
8. Stockholders’ equity
Common Stock
During the year ended December 31, 2017, the Company issued 453,209 net shares of common stock upon exercise of stock options. Stock options to purchase 182,471 shares of common stock were net exercised resulting in the net issuance of 83,491 shares of common stock and stock options to purchase 369,718 shares of common stock were exercised for cash, providing cash proceeds of $0.8 million. Furthermore, during the year ended December 31, 2017, the Company issued 1,505,026 shares of common stock due to warrant exercises. Net exercise of 89,330 shares of common stock underlying the warrants resulted in the issuance of 49,127 shares of common stock. Additionally, 1,455,899 were exercised resulting in cash proceeds of $3.3 million. The Company had no such net issuances of common stock during the year ended December 31, 2018.
Each share of the Company’s common stock is entitled to one vote and all shares rank equally as to voting and other matters. Dividends may be declared and paid on the common stock from funds legally available therefor, if, as and when determined by the Board of Directors.
Underwritten Public Offering
In February 2018, the Company completed an underwritten public offering of its common stock in which it sold 6,325,000 shares of its common stock, including the exercise in full by the underwriters of their option to purchase an additional 825,000 shares of its common stock, to the public at a price of $8.00 per share. The offering was completed under the shelf registration statement that was filed on Form S-3 and declared effective by the SEC on August 3, 2016. Net proceeds of the underwritten public offering, after deducting the underwriting discounts and commissions, were $47.6 million, excluding offering expenses of approximately $0.4 million incurred by the Company.
Private Placement and Preferred Stock
In June 2016, the Company entered into a securities purchase agreement, or the Securities Purchase Agreement, for a private placement of the Company’s securities with a select group of institutional investors, or the 2016 PIPE. The 2016 PIPE sale transaction, by the Company, consisted of 8,188,804 units at a price of $2.015 per unit for gross proceeds, to the Company, of approximately $16.5 million. After deducting for placement agent fees and offering expenses, the aggregate net proceeds from the private placement was approximately $15.3 million.
Each unit consisted of (i) one share of the Company’s common stock or non-voting series A convertible preferred stock, or the Series A Preferred Stock, which are convertible into one-thousand shares of common stock, (ii) one warrant to purchase 0.4 shares of common stock at an exercise price of $2.00 per share and (iii) one warrant to purchase 0.2 shares of common stock at an exercise price of $3.00 per share. The warrants will be exercisable for a period of five years from the date of issuance. Each share of Series A Preferred Stock was issued at a price of $2.015 per share, and is convertible into 1,000 shares of common stock, provided the holder and/or its affiliates do not own greater than 9.99% of the total number of Pieris common stock then outstanding. The Series A Preferred Stock has a par value of $0.001 per share, has no registration or voting rights, and holders are entitled to receive dividends on a pari passu basis with the Company´s common stock, when, and if declared. In event of a true liquidation or winding down of the business, holders of Series A Preferred Stock will be paid prior to the holders of common stock. In connection with the 2016 PIPE, the Company issued 3,225,804 shares of common stock and 4,963 shares of Series A Preferred Stock to the 2016 PIPE investors.
The Company had 2,907 and 4,963 shares of preferred stock both issued and outstanding during the years ended December 31, 2018 and 2017, respectively.
Preferred Share Exchange, Biotechnology Value Fund
On January 30, 2019, the Company and BVF entered into an the Exchange Agreement, pursuant to which BVF agreed to exchange, or the Exchange, an aggregate of 5,000,000 shares of the Company’s common stock owned by BVF for an aggregate of 5,000 shares of the Company’s newly-designated series B convertible preferred stock, par value $0.001 per share, or the Series B Preferred Stock. The Exchange closed on February 1, 2019.
The Series B Preferred Stock has substantially the same terms as the Company’s Series A Preferred Stock currently held by entities affiliated with BVF. The shares of Series B Preferred Stock issued in the Exchange are convertible into an aggregate of 5,000,000 shares of common stock provided the holder and/or its affiliates do not own greater than 9.99% of the total number of Pieris common stock then outstanding.
As of the date of the Exchange Agreement, BVF represented to the Company that it beneficially owned 7,457,921 shares of common stock, representing approximately 13.77% of the shares of common stock outstanding as of such date. In addition, BVF holds 2,907 shares of Series A Preferred Stock.
9. Net Loss per Share
Basic net loss per share is calculated by dividing net income (loss) by the weighted average shares outstanding during the period, without consideration for common stock equivalents. Diluted net loss per share is calculated by adjusting weighted average shares outstanding for the dilutive effect of common stock equivalents outstanding for the period, determined using the treasury-stock and if-converted methods. For purposes of the diluted net loss per share calculation, preferred stock, stock options, and warrants are considered to be common stock equivalents but have been excluded from the calculation of diluted net loss per share, as their effect would be anti-dilutive for all periods presented. Therefore, basic and diluted net loss per share were the same for all periods presented.
For the years ended December 31, 2018 and 2017, and as calculated using the treasury stock method, approximately 14.7 million and 15.9 million of weighted average shares, respectively, were excluded from the calculation of diluted weighted average shares outstanding as their effect was antidilutive.
10. Stock and Employee Benefit Plans
Employee, Director and Consultant Equity Incentive Plans
At the Annual Shareholder Meeting, held on July 24, 2018, the shareholders approved the 2018 Employee, Director and Consultant Equity Incentive Plan, or the 2018 Plan. Upon approval of the 2018 Plan, the 2016 Employee, Director and Consultant Equity Incentive Plan, or the 2016 Plan, was terminated and no additional awards will be made thereunder, however, all outstanding awards under the 2016 Plan will remain in effect. The 2018 Plan, similar to the 2016 Plan, provided for the grant of stock options, restricted and unrestricted stock awards, and other stock-based awards to employees of the Company, non-employee directors of the Company, and certain other consultants performing services for the Company as designated by either the Board of Directors or the compensation committee of the Board of Directors. Previously, upon approval of the 2016 Plan, the 2014 Employee, Director and Consultant Equity Incentive Plan, or the 2014 Plan, was terminated and no additional awards will be made thereunder, however, all outstanding awards under the 2014 Plan will remain in effect. The 2016 Plan, similar to the 2014 Plan, provided for the grant of stock options, restricted and unrestricted stock awards, and other stock-based awards to employees of the Company, non-employee directors of the Company, and certain other consultants performing services for the Company as designated by either the Board of Directors or the compensation committee of the Board of Directors.
There were approximately 86,000 shares remaining and available for grant under the 2016 Plan that terminated pursuant to the approval of the 2018 Plan. The 2018 Plan permits the Company to issue up to 9,975,000 shares, including 3,000,000 shares reserved for issuance pursuant to the 2018 Plan and up to 6,975,000 additional shares which may be issued if awards outstanding under the Registrant’s 2016 Plan are canceled or expire.
The Company’s stock options have a maximum term of 10 years from the date of grant. Stock options granted may be either incentive stock options or nonqualified stock options and the exercise price of stock options must be at least equal to the fair market value of the common stock on the date of grant. The Company’s general policy is to issue common shares upon the exercise of stock options.
The Company estimates the fair value of each stock award on the grant date using the Black-Scholes option-pricing model based on the following assumptions:
|
| | | | | | |
| | Years Ended December 31, |
| | 2018 | | 2017 |
Risk free interest rate | | 2.40% - 3.06% |
| | 1.77% - 2.22% |
|
Expected term (in years) | | 4.75 - 5.73 |
| | 5.00 - 5.73 |
|
Dividend yield | | — |
| | — |
|
Expected volatility | | 77.1% - 80.5% |
| | 75.1% - 78.9% |
|
The weighted-average fair value of the 1,881,660 and 1,873,047 options granted during the years ended December 31, 2018 and 2017 was $5.04 and $2.47, respectively. As of December 31, 2018, there were 2,830,034 shares available for future grant under the 2018 Plan.
The following table summarizes stock option activity for employees and nonemployees:
|
| | | | | | | | | | | | |
| Number of Options | | Weighted- Average Exercise Price | | Weighted- Average Remaining Contractual Life | | Aggregate Intrinsic Value (in thousands) |
Outstanding, December 31, 2017 | 5,443,485 |
| | $ | 2.33 |
| | | | $ | 27,822 |
|
Granted | 1,881,660 |
| | 7.59 |
| | | | — |
|
Exercised | (190,019 | ) | | 2.09 |
| | | | 861 |
|
Canceled | (285,079 | ) | | 3.28 |
| | | | 519 |
|
Outstanding, December 31, 2018 | 6,850,047 |
| | $ | 3.76 |
| | 7.68 years | | $ | 3,257 |
|
Vested or expected to vest, December 31, 2018 | 6,850,047 |
| | $ | 3.76 |
| | 7.68 years | | $ | 3,257 |
|
Exercisable, December 31, 2018 | 3,796,264 |
| | $ | 2.31 |
| | 6.82 years | | $ | 2,522 |
|
Periodically, the Company grants inducement options, which are awards outside of approved stock option plans, and which are material awards to the executive officers or other personnel entering senior leadership roles with the Company. The terms of inducement option awards were substantially the same as those issued under our 2016 Plan. These awards are excluded from the table above. The following table summarizes stock option activity for these inducement options (in thousands):
|
| | | | | | | | | | | | |
| Number of Options | | Weighted- Average Exercise Price | | Weighted- Average Remaining Contractual Life | | Aggregate Intrinsic Value (in thousands) |
Outstanding, December 31, 2017 | 1,531,250 |
| | $ | 3.62 |
| | | | $ | 6,015 |
|
Exercised | (406,250 | ) | | $ | 1.45 |
| | | | $ | 2,530 |
|
Outstanding, December 31, 2018 | 1,125,000 |
| | $ | 4.41 |
| | 7.75 years | | $ | — |
|
Vested or expected to vest | 950,000 |
| | $ | 4.14 |
| | 7.57 years | | $ | — |
|
Exercisable, December 31, 2018 | 590,625 |
| | $ | 3.94 |
| | 7.26 years | | $ | — |
|
The compensation expense with all inducement options was $0.7 million of which $0.4 million is included in research and development expense and $0.3 million is included in general and administration expense for the year ended December 31, 2018. The compensation expense for inducement options for the year ended December 31, 2017 was $0.8 million of which $0.3 million is included in research and development expense and $0.5 million is included in general and administration expense.
As of December 31, 2018, the total unrecognized compensation cost related to non-vested awards was $10.8 million of which $1.7 million are for inducement options. The Company expects to recognize the compensation cost over a remaining weighted-average period of 2.78 years.
Employee Stock Purchase Plans
Also at the Annual Shareholder Meeting, held on July 24, 2018, the shareholders approved the 2018 Employee Stock Purchase Plan, or the 2018 ESPP. The 2018 ESPP provides eligible employees with the opportunity, through regular payroll deductions, to purchase shares of the Company’s common stock at 85% of the lower closing market price of the common stock at the beginning date or ending date of each purchase period. The plan includes two six-month purchase periods per year in June and December. The first enrollment date into the plan was December 1, 2018 and no purchase have been made to date under the plan. The Company has reserved 500,000 shares of common stock for the administration of the 2018 ESPP. The fair value of shares expected to be purchased under the 2018 ESPP using the Black-Scholes model with the following assumptions:
|
| | | |
| | Years Ended December 31, |
| | 2018 |
Risk free interest rate | | 2.48 | % |
Expected term (in years) | | 0.5 |
|
Dividend yield | | — |
|
Expected volatility | | 57.57 | % |
Total stock-based compensation expense is recorded in operating expenses based upon the functional responsibilities of the individuals holding the respective options as follows (in thousands):
|
| | | | | | | | |
| | Years Ended December 31, |
| | 2018 | | 2017 |
Research and development | | $ | 1,984 |
| | $ | 844 |
|
General and administrative | | 2,959 |
| | 2,202 |
|
Total stock-based compensation | | $ | 4,943 |
| | $ | 3,046 |
|
11. License and Transfer Agreement
TUM License
The Company and TUM initiated discussions in the second quarter of 2018 to clarify, expand and restructure the research and licensing agreement with TUM, the TUM License, including the parties’ obligations under such license agreement. The TUM License assigns or exclusively licenses to the Company certain intellectual property related to the Company's Anticalin platform technology. The parties' recent discussions relate to revised commercial terms and to re-initiating additional collaborations between faculty at TUM and Pieris. While an amended and restated license agreement has not yet been completed, we intend to enter into such an amendment. The Company recorded the probable expected impact of the amendment in research and development expense as of December 31, 2018, which is an increase in our financial obligations associated with the TUM License of approximately $2.3 million, for amounts that would be due in 2019 for 2018 and 2017 sub-licensing activities. These discussions may also lead to an increase in the Company's collaborative research activities with TUM.
12. Commitments and Contingencies
Leases
The Company leases office and laboratory space in Freising, Germany as well as office space in Boston, Massachusetts. In Freising, the Company leases approximately 19,000 square feet of office and laboratory space under four agreements, including three leases for space on three floors of the same building and a letter agreement for additional conference room space within the building. Two of the lease agreements can be terminated by the Company at the end of any quarter with eight months' notice. The other lease agreement will terminate on January 31, 2020 and the letter agreement will terminate on December 31, 2019. In August 2015, the Company entered into the Sublease to lease approximately 3,950 square feet in Boston, Massachusetts. The Sublease expires on February 27, 2022 or such earlier date pursuant to the termination provisions of the Sublease.
In October 2018, Pieris GmbH entered into a new lease for office and laboratory space located in Hallbergmoos, Germany. Under the Lease Agreement, Pieris GmbH will rent approximately 105,000 square feet, of which approximately 96,400 square feet is expected to be delivered by the lessor in the fourth quarter of 2019 and approximately 8,600 square feet is expected to be delivered by the lessor by May 2020. An additional approximately 22,300 square feet is expected to be delivered by the lessor by October 2024. Pieris GmbH has a first right of refusal to lease an additional approximate 13,400 square feet. Pieris GmbH intends to move its operations currently conducted in Freising, Germany to the new leased property.
The Lease Agreement is contingent on the lessor obtaining appropriate building permits from governmental authorities and either party may rescind the Lease Agreement if the lessor does not obtain such permits within a specified period of time. The Lease Agreement provides for an initial term of 150 months, commencing on the date the lessor first delivers the leased property to Pieris GmbH as agreed under the lease agreement. Pieris GmbH also has an option to extend the term of the Lease Agreement for two additional 60-month periods. Pieris GmbH may sublease space within the leased property with lessor’s consent, which may not be unreasonably withheld.
Monthly base rent for the initial 105,000 square feet of the leased property, including parking spaces, will total approximately $0.2 million per month, which amount shall be adjusted starting on the second anniversary of the commencement date by an amount equal to the German consumer price index. In addition to the base rent, Pieris GmbH is also responsible for certain administrative and operational costs in accordance with the terms of the Lease Agreement. Pieris GmbH provided a security deposit of $0.8 million as of December 31, 2018. The Company will serve as a guarantor for the Lease Agreement.
Pieris GmbH and the lessor are each entitled to terminate the Lease Agreement for due cause. Specifically, the lessor may terminate for Pieris GmbH’s default on rent payments beyond certain amounts, noncompliance with major obligations under the Lease Agreement, and certain bankruptcy and insolvency events.
The Company records rent expense on a straight-line basis over the lease term period. The Company recognized rent expense under its existing lease agreements in an amount of $0.5 million and $0.5 million for the years ended December 31, 2018 and 2017, respectively.
The Company’s contractual commitments of the non-cancellable portion under these operating leases as of December 31, 2018 are as follows (in thousands):
|
| | | |
| Total |
2019 | $ | 613 |
|
2020 | 2,460 |
|
2021 | 2,464 |
|
2022 | 2,347 |
|
2023 | 2,365 |
|
Thereafter | 25,772 |
|
Total minimum lease payments | $ | 36,021 |
|